Abstract
Background
Cushing’s syndrome (CS) is a rare clinical condition caused by excessive cortisol secretion from adrenal glands. CS is associated with increased mortality and morbidity; therefore, a prompt diagnosis and an effective therapeutic approach are strongly necessary to improve the patient’s clinical management. The first-line treatment for CS is surgery, while medical treatment has historically played a minor role. However, thanks to the availability of novel compounds, the possibility of improving hypercortisolism control using different drug combinations emerged.
Purpose
No absolute recommendations are available to guide the therapeutic choice for patients with CS and, consequently, the awareness of unmet needs in CS management is growing. Although new data from clinical trials are needed to better define the most appropriate management of CS, an expert consensus approach can help define unmet needs and optimize the current CS management and treatment.
Methods
Twenty-seven endocrinologists from 12 Italian regions, working among the main Italian referral centers for hospital endocrinology where they take care of CS patients, were involved in a consensus process and used the Delphi method to reach an agreement on 24 statements about managing CS patients.
Results
In total, 18 statements reached a consensus. Some relevant unmet needs in the management of CS were reported, mainly related to the lack of a pharmacological treatment successful for the majority of patients.
Conclusion
While acknowledging the difficulty in achieving complete disease control, a significant change in CS management requires the availability of medical treatment with improved efficacy and safety over available therapeutic options at the time of the current study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s syndrome (CS) is a rare clinical condition caused by excessive cortisol secretion from the adrenal glands [1]. CS has an incidence of 1.5/1,000,000 inhabitants per year and a prevalence of nearly 60/1,000,000 inhabitants in Europe [2]. In approximately 80% of cases, CS is a consequence of an adrenocorticotrophin (ACTH) hypersecretion (ACTH-dependent CS), generally due to an ACTH-secreting pituitary tumor (pituitary-dependent CS or Cushing’s disease [CD], 70%), and, rarely, to an ACTH-secreting, or corticotrophin-releasing hormone-secreting, extra-pituitary tumor (ectopic CS, 10%) [3]. In the remaining 20% of cases, CS is a direct consequence of autonomous cortisol overproduction by the adrenal glands (ACTH-independent CS, adrenal CS) due to unilateral or bilateral adrenal diseases [1, 4, 5].
CS is associated with increased mortality, mostly attributable to cardiovascular complications and severe infections, as well as increased morbidity. The main comorbidities associated with CS include metabolic syndrome, cardiovascular diseases, immune disorders, musculoskeletal damage, neuropsychiatric diseases, impairment of reproductive and sexual function, together with dermatological manifestations, and suppression of pituitary function [5,6,7,8,9,10,11,12,13,14,15]. The entire cohort of these clinical complications substantially impairs the quality of life [5, 7,8,9,10,11].
A prompt diagnosis and an effective multidisciplinary therapeutic approach are strongly necessary to improve clinical picture and quality of life of patients with CS [10, 12]. Treatment goals include the normalization of cortisol levels, the reversion of clinical signs and symptoms, the prevention or improvement of concomitant comorbidities, the control of tumor growth, the long-term control of the disease without recurrence, and the restoration of normal mortality [10, 12].
The first-line treatment for CS is represented by surgery, aimed at removing the responsible tumor with consequent normalization of cortisol secretion and recovery of clinical syndrome [10, 16]. However, pituitary surgery, the main treatment of CD, is not effective in at least one-third of patients due to persistence or recurrence of the disease, therefore requiring a second therapeutic approach [10, 16]. Second-line treatments strictly depend on CS etiology and may include second pituitary surgery, medical treatment, radiotherapy and/or chemotherapy, and adrenal surgery [10].
Medical treatment has historically played a minor role in CS management. However, it has been acquiring a more important role in different steps of the treatment schedule, thanks to the availability of novel compounds and the employment of drugs previously used with different indications [5, 10, 16].
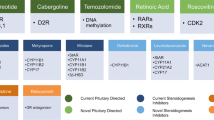
Particularly, medical treatment may be advocated before surgery, as preoperative treatment, especially in patients with severe CS, to control cortisol excess and improve the clinical picture [10]. Alternatively, it may be recommended as adjuvant treatment in patients with persistent or recurrent disease, or as bridging treatment before or after pituitary radiotherapy, while awaiting its definitive effects, or, lastly, as a primary alternative treatment in case of non-visible pituitary tumors at imaging procedures, lack of indications or contraindications to surgery, or surgery refusal [10]. The spectrum of available drugs to manage CS includes three main categories: (1) pituitary-directed drugs; (2) adrenal-directed drugs or steroidogenesis inhibitors; (3) glucocorticoid receptor-directed drugs [5, 16]. The main features of available drugs are summarized in Table 1.
The availability of different drugs has raised the possibility of combined treatment, using drugs acting at different levels to improve hypercortisolism control and safety profile of the single drugs [5, 16]. Nevertheless, no absolute recommendations are available to guide this therapeutic choice [17]. On this basis, the awareness of unmet needs is growing [18].
Although new data from clinical trials are needed to better define the most appropriate management of CS, an expert consensus approach, involving endocrinologists from the centers of excellence for pituitary tumors [19, 20], may help define unmet needs regarding CS and, thus, optimizing current management and treatment, mainly considering recently published guidelines and new scientific evidence [16].
To this aim, a group of Italian endocrinologists, working among the main referral centers for hospital endocrinology, was involved in a consensus process, using the Delphi method, to reach an agreement on a list of statements on the management and treatment of patients with CS concerning the Italian scenario. This manuscript presents and critically discusses the results of this consensus activity.
Methods
Based on the literature review and clinical experience, the authors defined the topics relevant for the analysis and the related statements through a series of online meetings held during April and May 2021. The authors developed an online survey, which was submitted to 3 experts among the authors (RP, CS, and AG) for approval and then was sent to 57 Italian endocrinologists belonging to referral centers for hospital endocrinology between June and September 2021. The large sample was chosen for the first invitation to achieve the entire cohort or at least the great majority of endocrinologists and endocrinology centers which could have expertise in CS management. A cover letter was sent to invited endocrinologists to explain the project and to invite them to answer the survey in case they have experience in CS management. The invited endocrinologists did not participate in the survey development. The invited endocrinologists, who replied to the invitation with acceptance to participate at the survey, represented the Delphi panel; the panelists used a dedicated online platform, and a timeline of 21 calendar days to respond to the survey. A further 7 days were granted after a reminder e-mail complete the process.
The survey included 24 statements based on the results coming from a literature review on the safety and clinical efficacy of current CS treatments and discussion among the authors. In some cases, the 24 statements were grouped and preceded by a brief introduction to frame the context and the rationale. The drugs reimbursed by the Italian national health system at the time of survey development, including pasireotide, ketoconazole, and metyrapone, were considered in formulating the pharmacological therapy statements. Noteworthy, osilodrostat obtained the reimbursement by the Italian national health system after the study completion.
The Delphi method was used to reach a consensus on the statements (scores on a 1–9 scale, with 1 indicating full disagreement and 9 indicating full agreement). A 70% threshold was set to define consensus, according to the most recent literature, meaning that strong disagreement or agreement was reached if at least 70% of participants had assigned scores in the range 1–3 or 7–9, respectively [21].
Statistical analysis
All data were analyzed with descriptive statistics.
Results
In total, 27 endocrinologists with expertise in the management of CS (47% of the identified sample) working in 12 different Italian regions participated in the survey, representing the Delphi panel. Among them, 16 (59%) reported following between 5 and 10 CS patients per year, 5 (19%) between 11 and 20 patients, 4 (15%) more than 20 patients, and 2 (7%) less than 5 patients.
The Delphi process was concluded in two rounds. In the first one, where 27 (100%) endocrinologists participated, agreement was reached on 15 (62%) out of the 24 proposed statements (numbers 1.1, 1.2, 1.3, 4.1, 5.4, 5.5, 6.1, 7.1, 7.2, 8.1, 8.2, 9.1, 9.2, 9.3, 10.1; Table 2). In the second round, where 23 (85.2%) of the initial 27 endocrinologists participated, an agreement was reached on an additional 3 (12.5%) statements (numbers 2.2, 3.1, and 5.2; Table 2). Consequently, at the end of the Delphi process, 18 (75%) statements out of 24 reached the consensus (Table 2); otherwise, 6 (25%) statements did not reach the consensus (numbers 2.1, 3.2, 3.3, 4.2, 5.1, and 5.3; Table 3).
Statements with agreement
Results from the Delphi panel highlighted the awareness of the current limitations of the surgical management of CD (agreement 81%) and bilateral adrenalectomy used in some forms of CS (78%), as well as the need for a periodic patient follow-up due to the risk of recurrence in the medium to long term (96%). Experts agreed on the need for new drug treatments with improved efficacy and safety to change the current management of CS (91%). Indeed, it was a shared opinion that current drug therapies allow a reduction in cortisol values but do not always determine its normalization (74%); it was also widely agreed that drug therapies for CS should have documented scientific evidence based on randomized clinical trials (RCTs, 78%), unlike certain drugs belonging to the category of steroidogenesis inhibitors reimbursed in Italy at the time of the current survey study. Regarding the current oral drug therapies for CS, most of the experts observed that the increase in drug dosage is very often paralleled by the increase in adverse events (AEs) (74%) and that it would be desirable to define new pharmacological therapies able to control the disease more quickly (70%) and for a longer period (86%) than current drug therapies. Experts agreed on the significant impact of the multiple daily administration on patient compliance and, consequently, on drug therapy adherence and effectiveness (74%). Experts shared the need to define new drug therapies able to reduce interruption rates (78%) and AEs that frequently affect clinical management mainly due to the requirement for supportive care, the patient’s perception of the therapy’s effectiveness and, therefore, the therapy adherence (81%). Experts recognized the impact of the escape phenomenon on disease control and patient management (92%) and, consequently, agreed on the need to define new therapeutic strategies to reduce escape rates (89%). Lastly, experts defined the need for a pharmacological treatment able to reduce both cortisol levels, signs, symptoms, and comorbidities, restore the rhythm of salivary cortisol, and reduce concomitant therapies (85%), allowing the medium-term to long-term control of the disease (89%), as well as the patient control, even after the treatment withdrawal (89%). All the experts encouraged the definition of patients’ pathways in terms of identifying centers of excellence and territorial networks (100%) (Table 2).
Statements with no consensus
Experts reported no consensus, due to failure to achieve the 70% threshold, on the availability of standard drug therapy for CS treatment (61% of agreement), on the rapid response time of the current drug therapies (35% of agreement), on the achievement of complete disease control with current drug therapies (30% of disagreement), on the opinion that CS therapies can be based on evidence from the clinical practice if data from RCTs are lacking (26% of disagreement), and on the satisfaction with the safety (35% of agreement) and efficacy profiles (17% of agreement) of the pharmacological therapies reimbursed at the time of the current survey study (Table 3).
Discussion
Nowadays, no absolute recommendations are available to guide the therapeutic management of CS patients and, consequently, the awareness of unmet needs is growing. To address these points, a group of Italian endocrinologists were involved in a consensus process using the Delphi method. A total of 27 endocrinologists, with expertise in the management of CS, from 12 out of the 20 different Italian regions, participated in this activity, thus representing the great majority of the Italian scenario. Nearly 80% of participants reported follow-up between 5 and 20 patients with CS per year, and 13% reported to follow-up more than 20 patients with CS per year, with only 7% of participants reporting to follow-up less than 5 patients. Considering that the number of patients with pituitary diseases is not large, and CS is a rare disease, these data suggest the proper selection of participants with a good grade of expertise. The results of the current study were able to highlight some relevant unmet needs, mainly related to the lack of an effective and safe pharmacological treatment successful for the majority of patients.
Surgical approach
Literature evidence suggests that pituitary surgery is widely considered the first-line treatment in CD management, even considering that an optimal success rate is reported especially among centers of excellence and may vary according to different factors, including patients’ characteristics, and preoperative visualization, size, and location of the pituitary tumor, as well as the surgeon experience [10, 16, 22, 23]. Consistent with this evidence, Delphi panel outcomes highlighted that the CD surgical management has some limitations (81% of agreement), mainly related to patients’ characteristics (refusal of surgery, comorbidities increasing the anesthesiologic/surgical risk, non-accessibility to all patients [15]), and to surgery issues, such as invisible or small size tumors, as well as unfavorable location or extrasellar expansion of the pituitary tumor. However, a minority of the respondents (11%) did not fully agree, likely based on recent data questioning the role of pre-surgical visualization or localization of the tumor on the surgical outcome [24]. Lastly, the unavailability of neurosurgeons with adequate expertise at the facility and, the possibility of medium-term to long-term relapse, requiring a mandatory periodical follow-up, even after a successful treatment, may impact on pituitary surgery success rate (96% of agreement).
Bilateral adrenalectomy was also recognized as a necessary treatment for CS in selected cases, especially in the case of failure of the remaining therapeutic approaches (78% of agreement). However, in line with literature evidence, experts agreed on the important limitations of this approach, such as permanent adrenal insufficiency leading to the need for lifelong glucocorticoid replacement therapy, with a high risk of developing acute adrenal crisis and corticotroph tumor progression (Nelson’s syndrome), particularly in the case of an evident pituitary tumor (78% of agreement) [10, 25].
Considering the current limitations of surgical approaches, implying that a significant proportion of patients are not eligible for these types of treatments or do not fully resolve the disease, most experts agreed that the availability of new drugs with improved efficacy and safety characteristics could change the CS management in a significant percentage of patients requiring alternative treatments to surgery (91% of agreement).
Pharmacological management
Results of the Delphi panel reported a wide agreement on relevant unmet medical needs in the pharmacological management of CS.
The measure of urinary free cortisol is currently the benchmark for controlling and monitoring CS. However, experts agreed that current pharmacological therapies for treating CS allow a reduction of cortisol values but do not always determine its normalization and, consequently, the patient control (74% of agreement). Moreover, experts agreed that most of the drugs reimbursed by the Italian national health system at the time of the current survey study, particularly the steroidogenesis inhibitors, do not have documented scientific evidence base due to the lack of randomized controlled trials (78% of agreement). Therefore, experts agreed on the need for new drug therapies with improved efficacy and safety profiles, especially drugs associated with rapid (70% of agreement) and prolonged (86% of agreement) disease control.
Although an acceptable overall safety profile for the different drugs approved for use in CD was reported [26,27,28], experts also agreed on the relevance of the issues related to treatment compliance, safety, and escape. Current oral pharmacological therapies require administration of multiple daily doses, with a significant impact on patient compliance and consequently on adherence and real effectiveness of therapy (74% of agreement). Moreover, certain drugs are characterized by a significant discontinuation rate due to AEs, which often increase in parallel with the increase in drug dosage (74% of agreement). Moreover, AEs may strongly influence clinical management, with the request of add-on supportive therapies, the patient’s perception of the treatment effectiveness, as well as therapy adherence (81% of agreement). Therefore, administration modalities should be improved, and AEs reduced, to significantly decrease interruption rates (78% of agreement). Finally, treatment escape has been recognized to impact disease control and patient management (92% of agreement). Therefore, its rate should be significantly reduced within new pharmacological therapies (89% of agreement).
To optimize the overall management of CS, new pharmacological therapies should be able to control not only cortisol levels but also clinical picture, in terms of signs, symptoms, and comorbidities, as well as potentially restore the circadian rhythm of cortisol and reduce concomitant therapies used to manage comorbidities (85% of agreement).
Due to the limitations of available drugs, CS medical treatment was considered a salvage therapy in most cases or a short-term bridge therapy. Nonetheless, experts agreed that new pharmacological therapies should safely enable disease control for the medium to long term (89% of agreement).
Lastly, in line with the most recent literature, the Delphi panel also unanimously agreed (100%) on the necessity to establish centers of excellence and territorial networks to optimize the management of CS patients based on multidisciplinary and individualized approaches, encouraging the development of specific “pathways” [19, 20]. For instance, in case of undetectable pituitary tumor or microadenoma, bilateral inferior petrosal sinus sampling (BIPSS) is suggested by current guidelines for CD diagnosis [16]. However, a recent study reported that in patients with pituitary microadenoma or non-visible tumor, a concordant positive response to non-invasive tests seems sufficient to diagnose CD, irrespective of MRI finding, and that the result of surgery is not influenced by the performance of BIPSS [24]. This finding is of relevance in particular for centers where BIPSS is not feasible.
Areas of debate
About one-third (35%) of the experts were uncertain about the existence of defined standard medical therapy for those patients not eligible for surgery or did not fully resolve after surgery, requiring alternative treatments to surgery. Notably, the most recent guidelines support using adrenal steroidogenesis inhibitors for rapid cortisol normalization among available therapeutic options [16]. This may reflect unresolved issues concerning their tolerability and efficacy, depending on individual patients’ characteristics as well as local availability and costs of each drug, which may lead to the choice of different adrenal steroidogenesis inhibitors to tailor therapy, that is recommended as the best therapeutic approach [16, 28]. For instance, although no rigorous data support the use of preoperative medical therapy, the use of adrenal steroidogenesis inhibitors can be considered if surgery is delayed [16, 19], at least in patients with severe disease who have potentially life-threatening complications. In addition, preoperative medical therapy could protect against proinflammatory and procoagulant states in the peri-operative phase [29,30,31]. On the other hand, adrenal steroidogenesis inhibitors do not directly target the pituitary tumor in the case of CD; therefore, they may not restore hypothalamic–pituitary–adrenal axis circadian rhythm [28], and their use may increase the risk of pituitary tumor enlargement [32]. Notably, a new meta-analysis highlighted the role of the somatostatin receptor ligand pasireotide, which is considered an alternative drug to steroidogenesis inhibitors, in the reduction of size in corticotroph pituitary tumor, strengthening the role of this treatment particularly in patients with tumor with an invasive behavior, progressive growth and/or extrasellar extension, with a low likelihood of surgical gross-total removal, or with large postoperative residual tissue [33]. Considering that up to 40% of CD patients achieved significant tumor shrinkage, this suggests a novel use of this medical treatment.
A lack of consensus was reported regarding the timing of the response to drug treatments. In detail, moderate agreement on the possibility of achieving short-time cortisol reduction with the current drug therapies was reached only by 61% of experts. This can be related to the absence of a reference time frame in the statement formulation. Indeed, treatment response can depend on the type of patient, gender, drug doses, disease duration, general state of health, or clinical history. Interestingly, this view can be changed by collecting additional real-life data, particularly for the new therapeutic options.
About one-third of respondents (30%) disagreed with the statement about the ability of current drug therapies to achieve complete disease control, intended as normalization of metabolic parameters, blood pressure, body weight, and bone mass; however, most respondents (65%) expressed moderate agreement with this statement, implying that perhaps complete control is obtained on only some of the reported parameters. Similarly, 74% of respondents were uncertain about the efficacy profiles of drugs reimbursed by the Italian national health system at the time of the survey study; about 9% were unsatisfied. The distribution of scores is probably affected, as above, by the presence of different outcome parameters to be considered in the global evaluation of the treatment outcome.
Although most of the experts agreed that therapies for CS must have documented scientific evidence based (e.g., randomized clinical trials; 78% of agreement), evidence from the clinical practice represented a matter of debate. A percentage of respondents (26%) appeared to disagree strongly; however, 56% considered this compromise acceptable. This suggests that, despite the peculiarities of this rare clinical condition, the robustness of the evidence should rely on both controlled trials and real-life data as well reflected by approval of all discussed drugs by the major drug agencies worldwide, including the FDA and EMA. Lastly, although it was recognized that an increase in AEs is very common with increasing drug dosages, most respondents (65%) were uncertain about their satisfaction with the efficacy and safety profile of currently reimbursed drug therapies (74% and 65%, respectively). However, only 35% of participants reported a high degree of satisfaction with the safety of the available therapeutic options.
Study limitations
A limitation of the current study relies on the fact that the survey was addressed to endocrinologists belonging to the main Italian referral centers for hospital endocrinology but only 27 of the 57 invited endocrinologists (47% of the identified sample) lastly participated at the survey. However, the relatively low participation to the survey did not impact the representativeness of the responses collected, since these centers are equally distributed throughout Italy and 60% of the Italian regions are represented, taking care of a different number of patients yearly, expression of the different realities among different referral centers. Notably, the relatively low participation to the survey may have been highly impacted by the emergency time due to the COVID-19 pandemic [34, 35]. It cannot be excluded that, in some instances, collected responses could have been influenced by possible misunderstandings of the questions, since no queries were issued to centers providing answers discordant from the majority of the respondents. However, in the opinion of the authors, discrepant results more likely depend on grey areas and conflicting published results and even recommendations in several areas of CS management.
Conclusion
The results of this consensus activity reported some relevant unmet needs in managing CS, mainly related to the lack of an effective and safe pharmacological treatment successful for the majority of patients. Up to date, the superiority of one drug over another could not be determined due to the lack of head-to-head controlled studies; moreover, the availability of a pharmacological treatment able to control hypercortisolism in the totality of CS patients and able to maintain the therapeutic effects during time is still an unmet clinical need [36]. Some pharmacological therapies in CS, such as ketoconazole and metyrapone, were approved in Europe based on small retrospective observational studies with mainly an empirical/pragmatic approach regarding dose finding [36]. Otherwise, the novel steroidogenesis inhibitor osilodrostat, recently achieving the reimbursement by the Italian health system, has been tested in prospective, randomized, phase III studies (LINC 3 and LINC 4), which showed significant and sustained normalization of cortisol levels vs placebo in CD naive patients or after unsuccessful pituitary surgery or irradiation (86% vs 29% and 77% vs 8% of patients in LINC 3 and LINC 4 study, respectively), associated with improved signs and comorbidities of CD and favorable safety and tolerability profiles [37, 38]. Considering the activity of osilodrostat, it appears to have the highest efficacy among the steroidogenesis inhibitors, followed by metyrapone and ketoconazole [5, 37], and, according to the authors’ opinion, it seems to have the appropriate profile to fill the gap in medical treatment.
In conclusion, while acknowledging the complexity of defining standard drug therapy for CS and the difficulty of achieving complete control of this clinical condition, a significant change in the CS therapeutic approach seems possible, considering the newly available treatment options. Moreover, the establishment of centers of excellence and territorial networks must be encouraged to optimize the management of CS patients based on multidisciplinary and individualized approaches.
Data availability
Materials described in the manuscript will be available to any researcher wishing to use them for non-commercial purposes, without breaching participant confidentiality. All datasets on which the conclusions of the paper rely will be available to readers. Datasets are presented in the main manuscript.
References
Pivonello R, De Martino MC, De Leo M, Simeoli C, Colao A (2017) Cushing’s disease: the burden of illness. Endocrine 56(1):10–18
Orphanet Report Series: Rare Diseases Collection. Prevalence and incidence of rare diseases: Bibliographic data. Prevalence, incidence, or number of published cases listed by diseases (in alphabetical order). 2020. https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf. Accessed 1 Jan 2022.
Melmed S, Kaiser UB, Lopes MB, Bertherat J, Syro LV, Raverot G, Reincke M, Johannsson G, Beckers A, Fleseriu M, Giustina A, Wass JAH, Ho KKY (2022) Clinical biology of the pituitary adenoma. Endocr Rev 8:bnac010. https://doi.org/10.1210/endrev/bnac010
Hayes AR, Grossman AB (2018) The ectopic adrenocorticotropic hormone syndrome: rarely easy, always challenging. Endocrinol Metab Clin North Am 47(2):409–425. https://doi.org/10.1016/j.ecl.2018.01.005
Pivonello R, Ferrigno R, De Martino MC, Simeoli C, Di Paola N, Pivonello C, Barba L, Negri M, De Angelis C, Colao A (2020) Medical treatment of Cushing’s disease: an overview of the current and recent clinical trials. Front Endocrinol (Lausanne) 11:648. https://doi.org/10.3389/fendo.2020.00648
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602. https://doi.org/10.1210/jc.2003-030871
Mazziotti G, Giustina A (2013) Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol 9(5):265–276. https://doi.org/10.1038/nrendo.2013.5
Zilio M, Barbot M, Ceccato F, Camozzi V, Bilora F, Casonato A, Frigo AC, Albiger N, Daidone V, Mazzai L, Mantero F, Scaroni C (2014) Diagnosis and complications of Cushing’s disease: gender-related differences. Clin Endocrinol (Oxf) 80(3):403–410. https://doi.org/10.1111/cen.12299
Huguet I, Ntali G, Grossman A, Karavitaki N (2015) Cushing’s disease—quality of life, recurrence and long-term morbidity. Eur Endocrinol 11(1):34–38
Pivonello R, De Leo M, Cozzolino A, Colao A (2015) The treatment of Cushing’s disease. Endocr Rev 36(4):385–486
Mazziotti G, Formenti AM, Adler RA, Bilezikian JP, Grossman A, Sbardella E, Minisola S, Giustina A (2016) Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 54(3):603–611. https://doi.org/10.1007/s12020-016-1146-8
Pivonello R, Isidori AM, De Martino MC, Newell-Price J, Biller BM, Colao A (2016) Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol 4(7):611–629. https://doi.org/10.1016/S2213-8587(16)00086-3
Formenti AM, Maffezzoni F, Doga M, Mazziotti G, Giustina A (2017) Growth hormone deficiency in treated acromegaly and active Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab 31(1):79–90. https://doi.org/10.1016/j.beem.2017.03.002
Barbot M, Zilio M, Scaroni C (2020) Cushing’s syndrome: overview of clinical presentation, diagnostic tools and complications. Best Pract Res Clin Endocrinol Metab 34(2):101380. https://doi.org/10.1016/j.beem.2020.101380
Frara S, Allora A, di Filippo L, Formenti AM, Loli P, Polizzi E, Tradati D, Ulivieri FM, Giustina A (2021) Osteopathy in mild adrenal Cushing’s syndrome and Cushing disease. Best Pract Res Clin Endocrinol Metab 35(2):101515. https://doi.org/10.1016/j.beem.2021.101515
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, Casanueva FF, Castinetti F, Chanson P, Findling J, Gadelha M, Geer EB, Giustina A, Grossman A, Gurnell M, Ho K, Ioachimescu AG, Kaiser UB, Karavitaki N, Katznelson L, Kelly DF, Lacroix A, McCormack A, Melmed S, Molitch M, Mortini P, Newell-Price J, Nieman L, Pereira AM, Petersenn S, Pivonello R, Raff H, Reincke M, Salvatori R, Scaroni C, Shimon I, Stratakis CA, Swearingen B, Tabarin A, Takahashi Y, Theodoropoulou M, Tsagarakis S, Valassi E, Varlamov EV, Vila G, Wass J, Webb SM, Zatelli MC, Biller BMK (2021) Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol 9(12):847–875. https://doi.org/10.1016/S2213-8587(21)00235-7
Albiger NM, Ceccato F, Zilio M, Barbot M, Occhi G, Rizzati S, Fassina A, Mantero F, Boscaro M, Iacobone M, Scaroni C (2015) An analysis of different therapeutic options in patients with Cushing’s syndrome due to bilateral macronodular adrenal hyperplasia: a single-centre experience. Clin Endocrinol (Oxf) 82(6):808–815. https://doi.org/10.1111/cen.12763
Störmann S, Schopohl J (2018) New and emerging drug therapies for Cushing’s disease. Expert Opin Pharmacother 19(11):1187–1200
Casanueva FF, Barkan AL, Buchfelder M, Klibanski A, Laws ER, Loeffler JS, Melmed S, Mortini P, Wass J, Giustina A, Pituitary Society, Expert Group on Pituitary Tumors (2017) Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): A Pituitary Society Statement. Pituitary 20(5):489–498. https://doi.org/10.1007/s11102-017-0838-2
Frara S, Rodriguez-Carnero G, Formenti AM, Martinez-Olmos MA, Giustina A, Casanueva FF (2020) Pituitary tumors centers of excellence. Endocrinol Metab Clin North Am 49(3):553–564. https://doi.org/10.1016/j.ecl.2020.05.010
Nasa P, Jain R, Juneja D (2021) Delphi methodology in healthcare research: How to decide its appropriateness. World J Methodol 11(4):116–129. https://doi.org/10.5662/wjm.v11.i4.116
Ferriere A, Tabarin A (2021) Cushing’s disease. Presse Med 50(4):104091
Brady Z, Garrahy A, Carthy C, O’Reilly MW, Thompson CJ, Sherlock M, Agha A, Javadpour M (2021) Outcomes of endoscopic transsphenoidal surgery for Cushing’s disease. BMC Endocr Disord 21(1):36. https://doi.org/10.1186/s12902-021-00679-9
Ferrante E, Barbot M, Serban AL, Ceccato F, Carosi G, Lizzul L, Sala E, Daniele A, Indirli R, Cuman M, Locatelli M, Manara R, Arosio M, Boscaro M, Mantovani G, Scaroni C (2022) Indication to dynamic and invasive testing in Cushing’s disease according to different neuroradiological findings. J Endocrinol Invest 45(3):629–637. https://doi.org/10.1007/s40618-021-01695-1
Sarkis P, Rabilloud M, Lifante JC, Siamand A, Jouanneau E, Gay E, Chaffanjon P, Chabre O, Raverot G (2019) Bilateral adrenalectomy in Cushing’s disease: altered long-term quality of life compared to other treatment options. Ann Endocrinol (Paris) 80(1):32–37. https://doi.org/10.1016/j.ando.2018.01.002
Mancini T, Porcelli T, Giustina A (2010) Treatment of Cushing disease: overview and recent findings. Ther Clin Risk Manag 6:505–516. https://doi.org/10.2147/TCRM.S12952
Ceccato F, Zilio M, Barbot M, Albiger N, Antonelli G, Plebani M, Watutantrige-Fernando S, Sabbadin C, Boscaro M, Scaroni C (2018) Metyrapone treatment in Cushing’s syndrome: a real-life study. Endocrine 62(3):701–711. https://doi.org/10.1007/s12020-018-1675-4
Puglisi S, Perotti P, Barbot M, Cosio P, Scaroni C, Stigliano A, Lardo P, Morelli V, Polledri E, Chiodini I, Reimondo G, Pia A, Terzolo M (2018) Preoperative treatment with metyrapone in patients with cushing’s syndrome due to adrenal adenoma. Endocr Connect 7(11):1227–1235. https://doi.org/10.1530/EC-18-0400
Stuijver DJ, van Zaane B, Feelders RA, Debeij J, Cannegieter SC, Hermus AR, van den Berg G, Pereira AM, de Herder WW, Wagenmakers MA, Kerstens MN, Zelissen PM, Fliers E, Schaper N, Drent ML, Dekkers OM, Gerdes VE (2011) Incidence of venous thromboembolism in patients with Cushing’s syndrome: a multicenter cohort study. J Clin Endocrinol Metab 96(11):3525–3532. https://doi.org/10.1210/jc.2011-1661
Valassi E, Feelders R, Maiter D, Chanson P, Yaneva M, Reincke M, Krsek M, Tóth M, Webb SM, Santos A, Paiva I, Komerdus I, Droste M, Tabarin A, Strasburger CJ, Franz H, Trainer PJ, Newell-Price J, Wass JA, Papakokkinou E, Ragnarsson O, ERCUSYN Study Group (2018) Worse health-related quality of life at long-term follow-up in patients with Cushing’s disease than patients with cortisol producing adenoma. Data from the ERCUSYN. Clin Endocrinol (Oxf) 88(6):787–798. https://doi.org/10.1111/cen.13600
Barbot M, Daidone V, Zilio M, Albiger N, Mazzai L, Sartori MT, Frigo AC, Scanarini M, Denaro L, Boscaro M, Casonato S, Ceccato F, Scaroni C (2015) Perioperative thromboprophylaxis in Cushing’s disease: what we did and what we are doing? Pituitary 18(4):487–493. https://doi.org/10.1007/s11102-014-0600-y
Pivonello R, Simeoli C, Di Paola N, Colao A (2022) Cushing’s disease: adrenal steroidogenesis inhibitors. Pituitary 25(5):726–732. https://doi.org/10.1007/s11102-022-01262-8
Mondin A, Manara R, Voltan G, Tizianel I, Denaro L, Ferrari M, Barbot M, Scaroni C, Ceccato F (2022) Pasireotide-induced shrinkage in GH and ACTH secreting pituitary adenoma: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 13:935759. https://doi.org/10.3389/fendo.2022.935759
Puig-Domingo M, Marazuela M, Giustina A (2020) COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine 68(1):2–5. https://doi.org/10.1007/s12020-020-02294-5
Giustina A, Marazuela M, Reincke M, Yildiz BO, Puig-Domingo M (2021) One year of the pandemic—how European endocrinologists responded to the crisis: a statement from the European Society of Endocrinology. Eur J Endocrinol 185(2):C1–C7. https://doi.org/10.1530/EJE-21-0397
Simões Corrêa Galendi J, Correa Neto ANS, Demetres M, Boguszewski CL, Nogueira VDSN (2021) Effectiveness of medical treatment of cushing’s disease: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 12:732240. https://doi.org/10.3389/fendo.2021.732240
Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, Gu F, Auchus R, Leelawattana R, Lee EJ, Kim JH, Lacroix A, Laplanche A, O’Connell P, Tauchmanova L, Pedroncelli AM, Biller BMK, LINC 3 investigators (2020) Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol 8(9):748–761. https://doi.org/10.1016/S2213-8587(20)30240-0
Gadelha M, Bex M, Feelders RA, Heaney AP, Auchus RJ, Gilis-Januszewska A, Witek P, Belaya Z, Yu Y, Liao Z, Chen Ku CH, Carvalho D, Roughton M, Wojna J, Pedroncelli AM, Snyder PJ (2022) Randomized trial of osilodrostat for the treatment of Cushing’s disease. J Clin Endocrinol Metab 107(7):e2882–e2895. https://doi.org/10.1210/clinem/dgac178
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94(1):115–122. https://doi.org/10.1210/jc.2008-1008
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM, Pasireotide B2305 Study Group (2012) A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366(10):914–924. https://doi.org/10.1056/NEJMoa1105743
Boscaro M, Bertherat J, Findling J, Fleseriu M, Atkinson AB, Petersenn S, Schopohl J, Snyder P, Hughes G, Trovato A, Hu K, Maldonado M, Biller BM (2014) Extended treatment of Cushing’s disease with pasireotide: results from a 2-year. Phase II study Pituitary 17(4):320–326. https://doi.org/10.1007/s11102-013-0503-3
Schopohl J, Gu F, Rubens R, Van Gaal L, Bertherat J, Ligueros-Saylan M, Trovato A, Hughes G, Salgado LR, Boscaro M, Pivonello R, Pasireotide B2305 Study Group (2015) Pasireotide can induce sustained decreases in urinary cortisol and provide clinical benefit in patients with Cushing’s disease: results from an open-ended, open-label extension trial. Pituitary 18(5):604–612. https://doi.org/10.1007/s11102-014-0618-1
Petersenn S, Salgado LR, Schopohl J, Portocarrero-Ortiz L, Arnaldi G, Lacroix A, Scaroni C, Ravichandran S, Kandra A, Biller BMK (2017) Long-term treatment of Cushing’s disease with pasireotide: 5-year results from an open-label extension study of a Phase III trial. Endocrine 57(1):156–165. https://doi.org/10.1007/s12020-017-1316-3
Trementino L, Michetti G, Angeletti A, Marcelli G, Concettoni C, Cardinaletti C, Polenta B, Boscaro M, Arnaldi G (2016) A single-center 10-year experience with pasireotide in Cushing’s disease: Patients’ characteristics and outcome. Horm Metab Res 48(5):290–298. https://doi.org/10.1055/s-0042-101347
Pivonello R, Arnaldi G, Scaroni C, Giordano C, Cannavò S, Iacuaniello D, Trementino L, Zilio M, Guarnotta V, Albani A, Cozzolino A, Michetti G, Boscaro M, Colao A (2019) The medical treatment with pasireotide in Cushing’s disease: an Italian multicentre experience based on “real-world evidence.” Endocrine 64(3):657–672. https://doi.org/10.1007/s12020-018-1818-7
Manetti L, Deutschbein T, Schopohl J, Yuen KCJ, Roughton M, Kriemler-Krahn U, Tauchmanova L, Maamari R, Giordano C (2019) Long-term safety and efficacy of subcutaneous pasireotide in patients with Cushing’s disease: interim results from a long-term real-world evidence study. Pituitary 22(5):542–551. https://doi.org/10.1007/s11102-019-00984-6
Fleseriu M, Iweha C, Salgado L, Mazzuco TL, Campigotto F, Maamari R, Limumpornpetch P (2019) Safety and efficacy of subcutaneous pasireotide in patients with Cushing’s disease: results from an open-label, multicenter, single-arm, multinational, expanded-access study. Front Endocrinol (Lausanne) 10:436. https://doi.org/10.3389/fendo.2019.00436
Lacroix A, Gu F, Gallardo W, Pivonello R, Yu Y, Witek P, Boscaro M, Salvatori R, Yamada M, Tauchmanova L, Roughton M, Ravichandran S, Petersenn S, Biller BMK, Pasireotide G2304 Study Group (2018) Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol 6(1):17–26. https://doi.org/10.1016/S2213-8587(17)30326-1
Fleseriu M, Petersenn S, Biller BMK, Kadioglu P, De Block C, T’Sjoen G, Vantyghem MC, Tauchmanova L, Wojna J, Roughton M, Lacroix A, Newell-Price J (2019) Long-term efficacy and safety of once-monthly pasireotide in Cushing’s disease: a phase III extension study. Clin Endocrinol (Oxf) 91(6):776–785. https://doi.org/10.1111/cen.14081
Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94(1):223–230. https://doi.org/10.1210/jc.2008-1533
Barbot M, Guarnotta V, Zilio M, Ceccato F, Ciresi A, Daniele A, Pizzolanti G, Campello E, Frigo AC, Giordano C, Scaroni C (2018) Effects of pasireotide treatment on coagulative profile: a prospective study in patients with Cushing’s disease. Endocrine 62(1):207–214. https://doi.org/10.1007/s12020-018-1669-2
Pivonello R, Pivonello C, Simeoli C, De Martino MC, Colao A (2022) The dopaminergic control of Cushing’s syndrome. J Endocrinol Invest 45(7):1297–1315. https://doi.org/10.1007/s40618-021-01661-x
Sonino N, Boscaro M, Paoletta A, Mantero F, Ziliotto D (1991) Ketoconazole treatment in Cushing’s syndrome: experience in 34 patients. Clin Endocrinol (Oxf) 35(4):347–352. https://doi.org/10.1111/j.1365-2265.1991.tb03547.x
Moncet D, Morando DJ, Pitoia F, Katz SB, Rossi MA, Bruno OD (2007) Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing’s syndrome. Medicina (B Aires) 67(1):26–31
Castinetti F, Morange I, Jaquet P, Conte-Devolx B, Brue T (2008) Ketoconazole revisited: a preoperative or postoperative treatment in Cushing’s disease. Eur J Endocrinol 158(1):91–99. https://doi.org/10.1530/EJE-07-0514
Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, Bihan H, Delemer B, Raverot G, Motte E, Philippon M, Morange I, Conte-Devolx B, Quinquis L, Martinie M, Vezzosi D, Le Bras M, Baudry C, Christin-Maitre S, Goichot B, Chanson P, Young J, Chabre O, Tabarin A, Bertherat J, Brue T (2014) Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab 99(5):1623–1630. https://doi.org/10.1210/jc.2013-3628
Shirley M (2021) Ketoconazole in Cushing’s syndrome: a profile of its use. Drugs Ther Perspect 37:55–64. https://doi.org/10.1007/s40267-020-00799-7
Jeffcoate WJ, Rees LH, Tomlin S, Jones AE, Edwards CR, Besser GM (1977) Metyrapone in long-term management of Cushing’s disease. Br Med J 2(6081):215–217. https://doi.org/10.1136/bmj.2.6081.215
Thorén M, Adamson U, Sjöberg HE (1985) Aminoglutethimide and metyrapone in the management of Cushing’s syndrome. Acta Endocrinol (Copenh) 109(4):451–457. https://doi.org/10.1530/acta.0.1090451
Verhelst JA, Trainer PJ, Howlett TA, Perry L, Rees LH, Grossman AB, Wass JA, Besser GM (1991) Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing’s syndrome. Clin Endocrinol (Oxf) 35(2):169–178. https://doi.org/10.1111/j.1365-2265.1991.tb03517.x
Valassi E, Crespo I, Gich I, Rodríguez J, Webb SM (2012) A reappraisal of the medical therapy with steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol (Oxf) 77(5):735–742. https://doi.org/10.1111/j.1365-2265.2012.04424.x
Daniel E, Aylwin S, Mustafa O, Ball S, Munir A, Boelaert K, Chortis V, Cuthbertson DJ, Daousi C, Rajeev SP, Davis J, Cheer K, Drake W, Gunganah K, Grossman A, Gurnell M, Powlson AS, Karavitaki N, Huguet I, Kearney T, Mohit K, Meeran K, Hill N, Rees A, Lansdown AJ, Trainer PJ, Minder AE, Newell-Price J (2015) Effectiveness of metyrapone in treating Cushing’s syndrome: a retrospective multicenter study in 195 patients. J Clin Endocrinol Metab 100(11):4146–4154. https://doi.org/10.1210/jc.2015-2616
Nieman LK, Boscaro M et al (2021) Metyrapone treatment in endogenous cushing’s syndrome: results at week 12 from PROMPT, a Prospective International Multicenter, Open-Label, Phase III/IV Study. J Endocr Soc 5(Suppl 1):A515. https://doi.org/10.1210/jendso/bvab048.1053
Patil CG, Veeravagu A, Prevedello DM, Katznelson L, Vance ML, Laws ER Jr (2008) Outcomes after repeat transsphenoidal surgery for recurrent Cushing’s disease. Neurosurgery 63:266–270
Hinojosa-Amaya JM, Cuevas-Ramos D, Fleseriu M (2019) Medical management of Cushing’s syndrome: current and emerging treatments. Drugs 79:935–956
Carroll TB, Peppard WJ, Herrmann DJ, Javorsky BR, Wang TS, Patel H, Zarnecki K, Findling JW (2018) Continuous etomidate infusion for the management of severe Cushing syndrome: Validation of a standard protocol. J Endocr Soc 3(1):1–12. https://doi.org/10.1210/js.2018-00269
Bertagna X, Pivonello R, Fleseriu M, Zhang Y, Robinson P, Taylor A, Watson CE, Maldonado M, Hamrahian AH, Boscaro M, Biller BM (2014) LCI699, a potent 11β-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing’s disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab 99(4):1375–1383. https://doi.org/10.1210/jc.2013-2117
Fleseriu M, Pivonello R, Young J, Hamrahian AH, Molitch ME, Shimizu C, Tanaka T, Shimatsu A, White T, Hilliard A, Tian C, Sauter N, Biller BM, Bertagna X (2016) Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary 19(2):138–148. https://doi.org/10.1007/s11102-015-0692-z
Gadelha M, Bex M, Feelders RA, Heaney AP, Auchus RJ, Gilis-Januszewska A, Witek P, Belaya Z, Yu Y, Liao Z, Ku CHC, Carvalho D, Roughton M, Wojna J, Pedroncelli AM, Snyder PJ (2022) Randomized trial of osilodrostat for the treatment of Cushing disease. J Clin Endocrinol Metab 107(7):e2882–e2895. https://doi.org/10.1210/clinem/dgac178
Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C, SEISMIC Study Investigators (2012) Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab 97(6):2039–2049. https://doi.org/10.1210/jc.2011-3350
DeGueme AM, King EE, Mirfakhraee S (2015) Medical treatment of Cushing’s disease with mifepristone: a clinical case series. Endocrine Rev. https://doi.org/10.1093/edrv/36.supp.1
Acknowledgements
The authors would like to thank all the Endocrinologists who took part to this project: Brunetti Antonio (Magna Graecia University, Calabria), Nuzzo Vincenzo (Del mare hospital, Naples), Scavuzzo Francesco (Cardarelli hospital, Naples), Appetecchia Maria Luisa (IRCCS IFO, Rome), Corsello Salvatore (A. Gemelli hospital, Rome), Gargiulo Patrizia (Umberto I hospital, Rome), Passeri Marina (Sant’Eugenio hospital, Rome), Stigliano Antonio (Sant’Andrea hospital, Rome), Ferone Diego (San Martino hospital, Genova), Arosio Maura (Ca’ Granda hospital, Milan), Dallabonzana Daniela (Ca’ Granda hospital, Milan), Lania Andrea (Humanitas hospital, Rozzano), Tanda Maria Laura (ASST Sette Laghi, Varese), Arvat Emanuela (Le Molinette hospital, Turin), Ghigo Ezio (Le Molinette hospital, Turin), Razzore Paola (Maugeri hospital, Turin), Terzolo Massimo (San Luigi hospital, Orbassano), Logoluso Francesco (Vittorio Emanuele II hospital, Bisceglie), Loviselli Andrea (Manserrato hospital, Cagliari), Belfiore Antonino (Garibaldi-Nesima hospital, Catania), Cannavo' Salvatore (Messina polyclinic, Messina), Giordano Carla (Palermo polyclinic, Palermo), Castagna Maria Grazia (Le Scotte hospital, Siena), Maggi Mario (Careggi hospital, Firenze), Peri Alessandro (Careggi hospital, Firenze), Marschang Peter (Bolzano hospital, Bolzano), Girelli Domenico (Borgo Roma hospital, Verona). Editorial assistance was provided by Simonetta Papa, PhD, Valentina Attanasio, and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by Recordati Rare Diseases.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. The work was made possible thanks to an unconditional contribution from Recordati Rare Diseases.
Author information
Authors and Affiliations
Contributions
All authors contributed to the definition and contextualization of the paper contents, critically edited the manuscript, and approved its final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
RP has been Principal Investigator of Clinical and/or Translational Research Studies for Novartis, Recordati Rare Diseases, HRA Pharma, Corcept Therapeutics, Cortendo AB-Strongbridge Biopharma; received research grants from Novartis, HRA Pharma, Cortendo AB-Strongbridge Biopharma; has been an occasional consultant for Novartis, HRA Pharma, Cortendo AB-Strongbridge Biopharma, Recordati Rare Diseases, Corcept Therapeutics; and has received fees and honoraria for presentations from Novartis and Recordati Rare Diseases beyond the confines of this work. CS has been Principal Investigator of Clinical and/or Translational Research Studies for Novartis, HRA Pharma, Shire, Cortendo AB-Strongbridge Biopharma, Pfizer, Recordati Rare Diseases; Co-investigator of Research Studies for Pfizer, Novartis; received research grants from Novartis, Pfizer, HRA Pharma; has been an occasional consultant for Novartis, Pfizer, Shire, HRA Pharma, Recordati Rare Diseases; and has received fees and honoraria for presentations from Novartis, Shire, Pfizer, Sandoz and Recordati Rare Diseases beyond the confines of this work. BP declares to have received in the last 5 years payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the following commercial sources: Allergan, Amgen, Astellas, Eli Lilly, Janssen Cilag, Nestle´ HS, Novartis, Novo Nordisk, Pfizer, Servier, Takeda, Teva; in addition, she received consulting fees from UCB. AG is consultant for Abiogen, Astellas, Ipsen, Novo. Pfizer, Recordati Rare Diseases, and Takeda and received research grants to the Institution from Ipsen, Novartis, Pfizer, Shire/Takeda.
Ethical statement
It is not required as this manuscript does not include details, images or videos related to patients/participants.
Research involving human participants and/or animals
The research does not involve human participants and/or animals, their data or biological material.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pivonello, R., Scaroni, C., Polistena, B. et al. Unmet needs on the current medical management of Cushing’s syndrome: results from a Delphi panel of Italian endocrinologists. J Endocrinol Invest 46, 1923–1934 (2023). https://doi.org/10.1007/s40618-023-02058-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02058-8