Abstract
Purpose
Laboratory, imaging, and pathological features of Graves’ disease (GD), although well characterized, have been barely correlated each other. Aim of the study was to link laboratory and ultrasound characteristics of GD with its pathological features.
Methods
We correlated laboratory and ultrasound data at the time of diagnosis in 28 consecutive GD patients who underwent thyroidectomy with their pathological features, i.e., lymphocytic infiltration and follicular hyperplasia (both classified as mild or severe).
Results
Thyroid volume correlated positively with the levels of FT4 (P = 0.002, r2 = 0.42), FT3 (P = 0.011, r2 = 0.22), autoantibodies to thyroglobulin (TgAbs) (P = 0.016, r2 = 0.32), autoantibodies to thyroid peroxidase (TPOAbs) (P = 0.011, r2 = 0.34) and the extent of lymphocytic infiltration (P = 0.006 comparing mild to severe lymphocytic infiltration) but not with the levels of autoantibodies to the thyrotropin receptor (TRAbs) and to follicular hyperplasia. Compared to subjects with mild lymphocytic infiltration, those with severe lymphocytic infiltration showed higher levels of TgAbs (316 vs 0.0 IU/mL, P < 0.0001) and TPOAbs (295 IU/mL vs 14 IU/mL, P < 0.0001) and similar levels of TRAbs (7.5 vs 13 IU/mL, P = 0.68). Compared to patients with mild, those with severe follicular hyperplasia had similar levels of TgAbs (76 vs 30 IU/mL, P = 0.31) and TPOAbs (251 IU/mL vs 45 IU/mL, P = 0.26) but higher levels of TRAbs (39 vs 7.2 IU/mL, P < 0.001).
Conclusion
In GD, TgAbs and TPOAbs levels correlate with the extent of lymphocytic infiltration, TRAbs levels with the degree of follicular hyperplasia. Thyroid volume, the main factor influencing the severity of hyperthyroidism, is related to lymphocytic infiltration and not to follicular hyperplasia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ disease (GD) is an autoimmune disorder which recognizes autoantibodies to the thyrotropin receptor (TRAbs) as the pathogenic driver [1,2,3]. At variance with many autoimmune diseases in which autoantibodies cause tissue damage, TRAbs stimulate the thyroid, inducing goiter and hyperthyroidism [2]. Patients with GD experience stigmata of hyperthyroidism, namely tachycardia, anxiety, insomnia and weight loss [1]. Currently, Graves’ orbitopathy occurred in up to 30% of patients whereas pretibial myxedema is reported in less than 10% of GD patients [4]. A diffuse goiter with high vascularization at neck ultrasound and high uptake at thyroid scintigraphy are the typical features of GD [1]. At histology, thyroid from GD patients is characterized by lymphocytic infiltration and follicular hyperplasia [5, 6]. In addition to TRAbs, the hallmark of the disease, autoantibodies to thyroglobulin (TgAbs) and to thyroid peroxidase (TPOAbs), which suggest a concomitant thyroiditis, are frequently detected in the serum of GD patients [7, 8]. The question whether GD always arises on a pre-existing thyroiditis or can develop because of the appearance of TRAbs on an otherwise normal thyroid remains unsettled.
Aim of the present study was to better characterize the features indicative of thyroid stimulation and those of lymphocytic thyroiditis in GD. For this purpose, we correlated the laboratory results and the ultrasound characteristics obtained at the time of diagnosis from a cohort of GD patients who underwent thyroidectomy with the histopathological features.
Methods
Study design and population
In 2017, seventy-four subjects underwent total thyroidectomy at the Endocrine Surgery Unit of University Hospital of Pisa because of GD. Among them, we identified the patients who had received a full evaluation, including blood tests and neck ultrasound, and the first diagnosis, prior to anti-thyroid treatment, at the Endocrinology Unit. Laboratory tests included measurement of FT4, FT3, TSH, TRAbs, TgAbs and TPOAbs. Twenty-eight patients matching the criteria were included in the study. The study was conducted in accordance with the ethical principles of Declaration of Helsinki. Data publication was approved by the local institutional review committee (Comitato Etico di Area Vasta Nord Ovest–CEAVNO). Patients were informed and gave their consent to participate in the study. All patients had been treated with anti-thyroid drugs prior to thyroidectomy.
Laboratory testing
Thyroid hormones and TSH were tested by immunoenzymatic assays (Ortho-clinical diagnostic Inc., Rochester, NY). Reference ranges were 8.0–18.0 ng/dL for FT4, 2.5–5.0 ng/L for FT3 and 0.4–4.0 mIU/L for TSH, respectively. TgAbs and TPOAbs were measured by AIA-Pack 2000 (Tosoh Corporation, Tokyo, Japan); analytic, functional and positive cut-offs were 6 IU/mL, 8 IU/mL and 30 IU/mL for TgAbs and 6 IU/mL, 10 IU/mL and 20 IU/mL for TPOAbs. TRAbs were measured by ELISA (ElisaRSR™ TRAb 3rd generation, Cardiff, UK) (positivity cutoff > 1.5 IU/mL).
Thyroid imaging
Neck ultrasound was performed by Technos (Esaote Biomedica, Genova, Italy), with a 7.5-MHz linear transducer. Thyroid volume was calculated using the ellipsoid volume formula. The normal thyroid volume in the Italian reference population is 4.0–12.1 mL in women and 6.0–16.0 mL in men [9]. According to their echoic pattern, thyroids were classified as slightly hypoechoic or markedly hypoechoic.
Pathology analysis
Hematoxylin and eosin-stained slides of thyroid specimens were collected and re-submitted to microscopic assessment by a pathologist expert in thyroid pathology who was masked to biochemical and imaging features of the patients and who carefully evaluated the histological features typical of Graves’ disease, i.e., follicular hyperplasia and lymphocytic infiltration. Sections 3–5 μm thick were deparaffinized in xylene, dehydrated, and processed using a diaminobenzidine detection system-BenchMark ULTRA IHC System (Ventana Medical Systems, Inc., Tucson, AZ). Lymphocytes were immunohistochemically identified by a monoclonal antibody—CD45 LCA-directed against an antigen located on their membrane. The number of lymphocytes was expressed as the sum of the cells in the most representative high power field (× 40 magnification) and lymphocytic thyroiditis was classified as mild, when the total number of lymphocytes was less than 50 per field or severe, when the total number was more than 50 per field [5, 10].
In absence of an universally accepted classification of follicular hyperplasia, the degree of follicular hyperplasia was categorized as mild, when thyroid follicles showed little variability in size and the pseudo-papillary projections were scattered and thin, or severe, when the thyroid tissue showed a diffuse crowding of the follicles, a variable degree of papillary structures, small-sized follicles and a scalloping of the colloid.
Statistical analysis
Statistical data analysis was performed using SPSS 21 (IBM Corp., Armonk, NY). Data are reported as mean ± SD or median with interquartile range (IQR), as indicated. The Shapiro–Wilk test was used to assess normality of data distribution of continuous variables. Statistical tests used to compare groups included Student’s t test for normally distributed variables and Mann–Whitney U tests for variables with skewed distribution. The Chi-squared or the Fisher exact tests were used to compare counts and frequencies between groups for categorical variables, as appropriate. Pearson’s (R) and Spearman’s (ρ) correlation coefficients were used to quantify association for Gaussian and skewed continuous variables, respectively.
Results
Characteristics of the study population
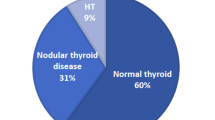
General characteristics, laboratory exams, ultrasound and pathology features of the study population are summarized in Table 1. At diagnosis, all subjects were hyperthyroid with high FT4 and FT3 and undetectable TSH levels. The time from the diagnosis of Graves’ disease to total thyroidectomy was 12.1 (range 6–24 months). Before surgery, 23 of 28 patients were treated with methimazole and 4 with propylthiouracil. Fourteen subjects had one or more thyroid nodules. No difference in the overall thyroid volume comparing patients with and without thyroid nodule(s) was observed. Twelve thyroid nodules meet the ultrasound criteria for fine needle aspiration, resulting benign. At histology, 10 were hyperplastic nodules and 2 follicular adenomas. Before surgery, 25 patients were treated with Lugol’s solution for a median of 5 days (range 3–11 days).
At pathology analysis, lymphocytic infiltration was mild in 16 and severe in 12 samples, follicular hyperplasia mild in 21 and severe in 7.
Correlation between laboratory tests at the time of diagnosis
The levels of FT4 and FT3 at the time of diagnosis did not correlate with the levels of TgAbs, TPOAbs and TRAbs (P > 0.05 for all correlations, data not shown).
While TgAbs and TPOAbs levels correlated each other (P = 0.006, r2 = 0.28) (Fig. 1A), no correlations were observed between the levels of TRAbs and those of TgAbs and TPOAbs (P > 0.05) (Fig. 1B–C).
Correlation between ultrasound and laboratory features at the time of diagnosis
Thyroid volume positively correlated with the levels of FT4 (P < 0.005, R2 = 0.42), FT3 (P = 0.011, r2 = 0.22), TgAbs (P = 0.016, r2 = 0.32) and TPOAbs (P = 0.011, r2 = 0.34) (Fig. 2A–D) but not with the levels of TRAbs (P = 0.49) (Fig. 2E).
The levels of both TgAbs and TPOAbs were higher in subjects with a markedly hypoechoic than in those with a slightly hypoechoic gland (220 vs 20 IU/mL, P < 0.001 for TgAbs and 264 vs 12 IU/mL, P = 0.001 for TPOAbs). At variance, no difference in the levels of TRAbs were found comparing patients with a markedly hypoechoic to those with a slightly hypoechoic thyroid (8.3 vs 15.8 IU/mL, P = 0.58).
Patients with a markedly hypoechoic gland showed a higher thyroid volume compared to those with a slightly hypoechoic gland (33.2 vs 18.3 mL, P = 0.02).
Correlations between the levels of thyroid hormones and thyroid antibodies, thyroid volume and histopathological features
FT4 and FT3 levels did not differ in patients with a mild lymphocytic infiltration compared to those with a severe lymphocytic infiltration (21.1 vs 32 ng/L, P = 0.5 for FT4 and 8.1 vs 13.2, P = 0.18 for FT3).
Compared to subjects with a mild lymphocytic infiltration, patients with a severe lymphocytic infiltration had higher levels of TgAbs (316 vs 0.0 IU/mL, P < 0.0001) (Fig. 3A) and TPOAbs (295 IU/mL vs 14 IU/mL, P < 0.0001) (Fig. 3B), whereas no difference was observed in TRAbs levels (7.5 vs 13 IU/mL, P = 0.68) (Fig. 3C). Compared to subjects with a mild lymphocytic infiltration, patients with a severe lymphocytic infiltration had a higher thyroid volume (37.8 vs 25.6 mL, P = 0.06) (Fig. 3D). The median time from the diagnosis to thyroidectomy was similar in patients with mild lymphocytic infiltration compared to those with severe lymphocytic infiltration (10.8 vs 11.7 months, P = 0.39).
Histopathological images of mild and severe lymphocytic infiltration obtained from GD patients and correlation to laboratory and thyroid volume at ultrasound. An example of mild and one of severe lymphocytic infiltration of the thyroid are shown. In the specimen with a mild lymphocytic infiltration, lymphocytes are small in number and restricted to the interstitial stroma between the thyroid follicles. In that with a severe lymphocytic infiltration, the lymphocytic infiltrate is more prominent and is associated with lymphoid aggregate and germinal center (hematoxylin and eosin staining, original magnification × 100). TgAbs (A), TPOAbs (B), TRAbs levels (C) and thyroid volume (D) observed at the time of diagnosis in subjects with a mild and in those with a severe lymphocytic infiltration are compared.
FT4 and FT3 levels did not differ between subjects with a severe hyperplasia compared to those with a mild hyperplasia (17.8 vs 22.5 ng/L, P = 0.34 for FT4 and 12.1 vs 10.2 for FT3, P = 0.52). Compared to subjects with a mild follicular hyperplasia, patients with a severe follicular hyperplasia had similar levels of TgAbs (76 vs 30 IU/mL, P = 0.31) (Fig. 4A) and TPOAbs (25.1 IU/mL vs 45 IU/mL, P = 0.26) (Fig. 4B) and higher levels of TRAbs (39 vs 7.2 IU/mL, P < 0.001) (Fig. 4C). Compared to subjects with a mild follicular hyperplasia, patients with a severe follicular hyperplasia had a similar thyroid volume (30 vs 26 mL, P = 0.95) (Fig. 4D). The median time from the diagnosis to thyroidectomy was similar in patients with a mild follicular hyperplasia compared to those with a severe follicular hyperplasia (11.2 vs 13.1 months, P = 0.48).
Histopathological images of mild and severe follicular hyperplasia obtained from GD patients and correlation to laboratory and thyroid volume at ultrasound. An example of mild and one of severe follicular hyperplasia of the thyroid are shown. In the specimen with mild follicular hyperplasia, thyroid follicles show little heterogeneity in size and pseudo-papillary projections are scattered and thin (hematoxylin and eosin staining, original magnification × 100). In that with severe follicular hyperplasia, papillary structures are exuberant and lined by cuboidal follicular cells showing round nuclei. TgAbs (A), TPOAbs (B), TRAbs levels (C) and thyroid volume (D) observed at the time of diagnosis in subjects with a mild and in those with a severe follicular hyperplasia are compared
Discussion
Graves’ disease is the most common cause of hyperthyroidism in iodine sufficient areas, affecting 3% of women and 0.5% of men during their lifetime [1, 11]. Clinically, it is characterized at onset by symptoms and signs of thyrotoxicosis [1]. Patients may also experience extrathyroidal manifestations, namely Graves’ ophthalmopathy and dermopathy (pretibial myxedema) [12, 13]. At laboratory tests, GD patients show high levels of FT4 and FT3 and undetectable levels of TSH [1]. TRAbs are positive in most subjects [14, 15], whereas TgAbs are positive in up to 80% and TPOAbs in 90% of patients [7]. At imaging, GD is characterized by a diffuse goiter with high vascularization at neck ultrasound and high uptake at thyroid scintigraphy [1]. At pathology, follicular hyperplasia, the hallmark of the disease, is associated with a variable degree of lymphocytic infiltration [5, 6].
Few studies have investigated the link between laboratory results, ultrasound features and the pathological characteristics of GD [16, 17]. We decided to correlate the laboratory and imaging findings collected at the time of diagnosis from a cohort of GD patients, who underwent thyroidectomy, with their histopathological features.
The male to female ratio and the mean age of the study population were similar to those previously reported [1, 11]. All patients were hyperthyroid at the time of diagnosis, with a typical high FT3/FT4 ratio. TRAbs were positive in all subjects whereas TgAbs were positive in 46% and TPOAbs in 78%, respectively. A positive correlation was found between the levels of TgAbs and those of TPOAbs, whereas no correlation was found between the levels of TgAbs and TPOAbs and those of TRAbs.
TgAbs and TPOAbs but, to our surprise, not TRAbs levels positively correlated with thyroid volume and the degree of hypoechogenicity. At pathology, we observed that the severity of lymphocytic infiltration correlated with TgAbs and TPOAbs levels, while the severity of hyperplasia was positively associated with TRAbs levels.
All these findings are in agreement with the notion that two distinct, but related factors are involved in the pathogenesis of GD. On one hand, TRAbs are related to the follicular hyperplasia typical of the disease [2, 18] On the other hand, a “thyroiditis background” characterized by a diffuse lymphocytic infiltration and high levels of TgAbs and TPOAbs in observed in most GD thyroid glands [5]. TRAbs are required for the onset of GD but their levels do not correlate with the degree of lymphocytic infiltration [2, 19]. In animal models of GD thyroid lymphocytic infiltration is seldom observed after immunization with the TSHR [2, 19,20,21]. At variance, a strong association between lymphocytic infiltration and the levels of TgAbs and TPOAbs has been demonstrated in different settings of thyroid disease in humans [10, 22]. It has been shown that in GD lymphocytic infiltration is usually inhomogeneous and that the follicular hyperplasia is more extensive in the areas with a higher lymphocytic infiltration, likely because of a different degree of antigenicity between follicles [23, 24].
The pathogenic events underlying TRAbs appearance are still unknown. In our cohort, most patients had a thyroiditis background (i.e., lymphocytic infiltration and positive TgAbs and TPOAbs). However, lymphocytic infiltration was correlated to TgAbs and TPOAbs but not to TRAbs levels. The phenotype of positive TRAbs, negative TgAbs, negative TPOAbs and absent lymphocytic infiltration was observed in few patients. The appearance of TRAbs associated to the onset of GD after thyroid injury due to subacute thyroiditis or treatment with 131iodine is a rare event [25, 26]. Our data suggest that GD usually arises on a thyroiditis, linked to serum TgAbs and TPOAbs. The onset of GD because of the appearance of TRAbs in the absence of a lymphocytic thyroiditis is a rare occurrence.
In line with this view, we observed that, at the time of the onset of the disease, goiter was mainly due to lymphocytic infiltration and not to follicular hyperplasia, as commonly stated [3]. Indeed, to our great surprise, we did not observe the previously reported correlation between TRAbs levels and thyroid volume [27, 28]. This discrepancy is likely due to a variation in the phenotypic appearance of Graves’ disease, nowadays diagnosed earlier and in milder forms than in the past [23, 29]. It is indeed reasonable that the extensive follicular hyperplasia reported by the oldest studies and its correlation with TRAbs levels were due to a delay in diagnosis, which enabled a prolonged stimulation of TRAbs until thyroidectomy [28, 29]. At variance, in our patients who were thyroidectomized early after diagnosis, lymphocytic infiltration was the main determinant of thyroid volume. For the same reason we did not observe the previously reported correlation between the levels of TRAbs and those of FT3 [28, 30]. An alternative explanation for the last finding is that the TBI (thyrotrophin binding index) assay we used measure blocking in addition to stimulating TRAbs, which are those directly inducing hyperthyroidism. However, this is unlikely, because in GD total TRAbs measured as TBI are tightly associated to stimulating TRAbs [31, 32].
Because thyroidectomy is usually proposed to subjects with high thyroid volume, not eligible to radioiodine therapy, one could argue that the present study is affected by the bias of having included only patients with a large goiter [33]. However, it is noteworthy that the median thyroid volume of patients of the cohort is not particularly high. In addition, although we did not find any association between the timing from the diagnosis to thyroidectomy and the histological features, we do not exclude that the duration of the therapy with anti-thyroid drugs may change the follicular hyperplasia and/or the lymphocytic infiltration. This issue could be accurately evaluate only analyzing a large series of GD patients undergone total thyroidectomy at different times from the diagnosis.
In conclusion, our study highlights that GD usually arises from a pre-existing lymphocytic thyroiditis whereas the onset of TRAbs in its absence is uncommon. We observed that TRAbs levels are strictly related to the degree of follicular hyperplasia, TgAbs and TPOAbs levels to the amount of lymphocytic infiltration. Nowadays thyroid volume, the main factor influencing the severity of thyrotoxicosis at diagnosis, is mainly due to lymphocytic infiltration rather than to follicular hyperplasia.
Data availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
Smith TJ, Hegedüs L (2016) Graves’ disease. N Engl J Med 375(16):1552–1565
Rapoport B, McLachlan SM (2007) The thyrotropin receptor in Graves’ disease. Thyroid 17(10):911–922
Davies TF (2013) Pathogenesis of Graves’ disease. In: Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text, Tenth Edition. p.356–69.
Schuh A, Ayvaz G, Baldeschi L, Baretić M, Bechtold D, Boschi A, Brix TH, Burlacu M-C, Ciric J, Covelli D et al (2023) Presentation of Graves’ orbitopathy within European group on graves’ orbitopathy (EUGOGO) centres from 2012 to 2019 (PREGO III). Br J Ophthalmol. https://doi.org/10.1136/bjo-2022-322442
LiVolsi VA, Baloch ZW (2018) The pathology of hyperthyroidism. Front Endocrinol (Lausanne) 9:737
Ippolito S, Piantanida E, Tanda ML, Caturegli P (2020) Graves’ disease insights from a review of the Johns Hopkins surgical pathology archive. J Endocrinol Invest 43(10):1519–1522
Beever K, Bradbury J, Phillips D, McLachlan SM, Pegg C, Goral A, Overbeck W, Feifel G, Smith BR (1989) Highly sensitive assays of autoantibodies to thyroglobulin and to thyroid peroxidase. Clin Chem 35(9):1949–1954
Rapoport B (1991) Thyroid-specific antigens in Basedow’s disease. Exp Clin Endocrinol 97(2–3):147–152
Rago T, Cantisani V, Ianni F, Chiovato L, Garberoglio R, Durante C, Frasoldati A, Spiezia S, Farina R, Vallone G et al (2018) Thyroid ultrasonography reporting: consensus of Italian thyroid association (AIT), Italian society of endocrinology (SIE), Italian society of ultrasonography in medicine and biology (SIUMB) and ultrasound chapter of italian society of medical radiology (SIRM). J Endocrinol Invest 41(12):1435–1443
Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A et al (2012) Lymphocytic thyroiditis on histology correlates with serum thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: impact on detection of serum thyroglobulin. J Clin Endocrinol Metab 97(7):2380–2387
Nystrom HF, Jansson S, Berg G (2013) Incidence rate and clinical features of hyperthyroidism in a long-term iodine sufficient area of Sweden (Gothenburg) 2003–2005. Clin Endocrinol (Oxf) 78(5):768–776
Bahn RS (2010) Graves’ ophthalmopathy. N Engl J Med 362(8):726–738
Dickinson AJ, Perros P (2001) Controversies in the clinical evaluation of active thyroid-associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol (Oxf) 55(3):283–303
Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N (2012) TSH receptor autoantibody immunoassay in patients with Graves’ disease: improvement of diagnostic accuracy over different generations of methods. Syst Rev Meta-Anal Autoimmun Rev 12(2):107–113
Barbesino G, Tomer Y (2013) Clinical review: clinical utility of TSH receptor antibodies. J Clin Endocrinol Metab 98(6):2247–2255
Spjut HJ, Warren WD, Ackerman LV (1957) Clinical-pathologic study of 76 cases of recurrent Graves’ disease, toxic (non-exophthalmic) goiter, and nontoxic goiter; does a relation exist between thyroid hyperplasia and struma lymphomatosa? Am J Clin Pathol 27(4):367–392
LiVolsi VA (1994) The pathology of autoimmune thyroid disease: a review. Thyroid 4(3):333–339
Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger. PP (2001) Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev 22(5):631–656
McLachlan SM, Rapoport B (2014) Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity. Endocr Rev 35(1):59–105
Shimojo N, Kohno Y, Yamaguchi K, Kikuoka S, Hoshioka A, Niimi H, Hirai A, Tamura Y, Saito Y, Kohn LD et al (1996) Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyrotropin receptor and a class II molecule. Proc Natl Acad Sci U S A 93(20):11074–11079
Kita M, Ahmad L, Marians RC, Vlase H, Unger P, Graves PN, Davies TF (1999) Regulation and transfer of a murine model of thyrotropin receptor antibody mediated Graves’ disease. Endocrinology 140(3):1392–1398
Caturegli P, De Remigis A, Rose NR (2014) Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmunity Rev. https://doi.org/10.1016/j.autrev.2014.01.007
Va L (1990) Surgical pathology of the thyroid. Saunders, Philadelphia, PA
Paschke R, Brückner N, Eck T, Schaaf L, Back W, Usadel KH (1991) Regional stimulation of thyroid epithelial cells in Graves’ disease by lymphocytic aggregates and plasma cells. Acta Endocrinol (Copenh) 125(5):459–465
Chiovato L, Santini F, Vitti P, Bendinelli G, Pinchera A (1994) Appearance of thyroid stimulating antibody and Graves’ disease after radioiodine therapy for toxic nodular goitre. Clin Endocrinol (Oxf) 40(6):803–806
Bartalena L, Bogazzi F, Pecori F, Martino E (1996) Graves’ disease occurring after subacute thyroiditis: report of a case and review of the literature. Thyroid 6(4):345–348
Bliddal H, Hegedüs L, Hansen JM, Bech K, van der Gaag R, Drexhage HA (1987) The relationships between serum T3 index, thyroid volume, and thyroid stimulating, TSH receptor binding and thyroid growth stimulating antibodies in untreated Graves’ disease. Clin Endocrinol (Oxf) 27(1):75–84
Wenzel KW, Lente JR (1983) Syndrome of persisting thyroid stimulating immunoglobulins and growth promotion of goiter combined with low thyroxine and high triiodothyronine serum levels in drug treated Graves’ disease. J Endocrinol Invest 6(5):389–394
Pinto W, Romaldini JH, Perini N, Santos RB, Villagelin D (2021) The change in the clinical presentation of Graves’ disease: a 30 years retrospective survey in an academic Brazilian tertiary center. Arch Endocrinol Metab 64(5):514–520
Hegedüs L, Hansen JM, Bech K, Kampmann JP, Jensen K, Andersen E, Hansen P, Karstrup S, Bliddal H (1984) Thyroid stimulating immunoglobulins in Graves’ disease with goitre growth, low thyroxine and increasing triiodothyronine during PTU treatment. Acta Endocrinol (Copenh) 107(4):482–488
Huang Y, Jin B, Huang Y, Dong A (2022) Consistency between thyrotropin receptor antibody (TRAb) and thyroid-stimulating antibody (TSAb) levels in patients with Graves disease. Lab Med 53(4):412–416
Autilio C, Morelli R, Locantore P, Pontecorvi A, Zuppi C, Carrozza C (2018) Stimulating TSH receptor autoantibodies immunoassay: analytical evaluation and clinical performance in Graves’ disease. Ann Clin Biochem 55(1):172–177
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN et al (2016) 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26(10):1343–1421
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. This work was supported by “Fondi di Ateneo”, University of Pisa to Francesco Latrofa.
Author information
Authors and Affiliations
Contributions
AB and FL planned the study. LT performed the pathological analysis. AB, FL, and LT analyzed the data. AB, FL and FS wrote the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of research reported. This work was supported by “Fondi di Ateneo”, University of Pisa to Francesco Latrofa.
Research involving human participants and/or animals
The reasarch involved human participants. Data publication was approved by the local institutional review committee (Comitato Etico di Area Vasta Nord Ovest–CEAVNO).
Informed consent
Patients were informed and gave their consent to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brancatella, A., Torregrossa, L., Viola, N. et al. In Graves’ disease, thyroid autoantibodies and ultrasound features correlate with distinctive histological features. J Endocrinol Invest 46, 1695–1703 (2023). https://doi.org/10.1007/s40618-023-02044-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02044-0