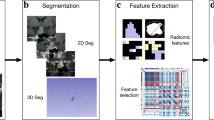
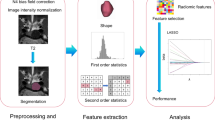
Abstract
Objective
Silent corticotroph adenomas (SCAs) are a subtype of nonfunctioning pituitary adenomas that exhibit more aggressive behavior. However, rapid and accurate preoperative diagnostic methods are currently lacking.
Design
The purpose of this study was to examine the differences between SCA and non-SCA features and to establish radiomics models and a clinical scale for rapid and accurate prediction.
Methods
A total of 260 patients (72 SCAs vs. 188 NSCAs) with nonfunctioning adenomas from Peking Union Medical College Hospital were enrolled in the study as the internal dataset. Thirty-five patients (6 SCAs vs. 29 NSCAs) from Fuzhou General Hospital were enrolled as the external dataset. Radiomics models and an SCA scale to preoperatively diagnose SCAs were established based on MR images and clinical features.
Results
There were more female patients (internal dataset: p < 0.001; external dataset: p = 0.028) and more multiple microcystic changes (internal dataset: p < 0.001; external dataset: p = 0.012) in the SCA group. MRI showed more invasiveness (higher Knosp grades, p ≤ 0.001). The radiomics model achieved AUCs of 0.931 and 0.937 in the internal and external datasets, respectively. The clinical scale achieved an AUC of 0.877 and a sensitivity of 0.952 in the internal dataset and an AUC of 0.899 and a sensitivity of 1.0 in the external dataset.
Conclusions
Based on clinical information and imaging characteristics, the constructed radiomics model achieved high preoperative diagnostic ability. The SCA scale achieved the purpose of rapidity and practicality while ensuring sensitivity, which is conducive to simplifying clinical work.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- SCA:
-
Silent corticotroph adenomas
- NFPA:
-
Nonfunctioning pituitary adenoma
- ACTH:
-
Adrenocorticotropic hormone
- MMs:
-
Multiple microcysts
- ICA:
-
Internal carotid artery
References
Lopes MJ, Bs AN (2017) The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol 134:521
Louis DN et al (2021) The 2021 who classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Horvath E et al (1980) Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Endocrine 98(3):617–638
Righi A et al (2016) The changing faces of corticotroph cell adenomas: the role of prohormone convertase 1/3. Endocrine 56(2):1–12
Raverot G et al (2010) Clinical, hormonal and molecular characterization of pituitary ACTH adenomas without (silent corticotroph adenomas) and with Cushing’s disease. Euro J Endocrinol. 163(1):35
Cheres AE et al (2017) Perioperative hypothalamic pituitary adrenal function in patients with silent. corticotroph adenomas 20(4):471–476
Yamada S et al (2007) A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 61(3):580
Cho HY et al (2010) Silent corticotroph adenomas have unique recurrence characteristics compared with other nonfunctioning. pituitary adenomas 72(5):648–653
Langlois F et al (2017) Clinical profile of silent growth hormone pituitary adenomas; higher recurrence rate compared to silent gonadotroph pituitary tumors a large single center experience. Endocrine 58:534
Arman J et al (2013) A comprehensive long-term retrospective analysis of silent corticotrophic adenomas vs hormone-negative adenomas. Neurosurgery 1:17–18
Jiang S, Zhu J, Feng M, Yao Y, Deng K, Xing B, Lian W, Wang R, Bao X (2021) Clinical profiles of silent corticotroph adenomas compared with silent gonadotroph adenomas after adopting the 2017 WHO pituitary classification system. Pituitary 24:564–573
Cazabat L et al (2014) Silent, but not unseen:Multi-microcystic aspect on T2-weighted MRI in Silent Corticotroph Adenomas. Clinical Endocrinol 81(4):566–572
Nishioka H, Inoshita N, Mete O, Asa SL, Hayashi K, Takeshita A, Fukuhara N, Yamaguchi-Okada M, Takeuchi Y, Yamada S (2015) The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol 26:349–355
He, W., et al., Development and Evaluation of Deep Learning-based Automated Segmentation of Pituitary Adenoma in Clinical Task. (9): p. 9.
Fan Y et al (2019) Machine learning-based radiomics predicts radiotherapeutic response in patients with acromegaly. Front Endocrinol (Lausanne) 10:588
Fan Y et al (2019) Preoperative noninvasive radiomics approach predicts tumor consistency in patients with acromegaly: development and multicenter prospective validation. Front Endocrinol (Lausanne) 10:403
Ugga L et al (2019) Prediction of high proliferative index in pituitary macroadenomas using MRI-based radiomics and machine learning. Front Endocrinol 61(12):1365–1373
Cuocolo R et al (2020) Prediction of pituitary adenoma surgical consistency: radiomic data mining and machine learning on T2-weighted MRI. Neuroradiology 62:1649
Ajp A et al (2020) A machine learning model to precisely immunohistochemically classify pituitary adenoma subtypes with radiomics based on preoperative magnetic resonance imaging. Sci Direct. 125:453
Bahuleyan B et al (2006) To assess the ability of MRI to predict consistency of pituitary macroadenomas. Sci Direct 20(5):324–326
Griethuysen JJMV et al (2017) Computational Radiomics System to Decode the Radiographic. Phenotype 77(21):e104–e107
Braileanu M et al (2019) Pre-operative MRI predictors of hormonal remission status post pituitary. adenoma resec 55:29–34
Hui Z, Hastie T (2012) elasticnet Elastic-Net for Sparse Estimation and Sparse PCA. Mach Learn 45:554
Breiman LJ (2001) Random forests. Mach Learn 45(1):5–32
Geurts P, Ernst D, Wehenkel LJML (2006) Extremely randomized trees 63(1):3–42
Quinlan JR (1986) Induction of decision trees. Mach Learn 1(1):81–106
Friedman JHJCSD (2002) Stochastic gradient boosting. Mach Learn 38(4):367–378
Schapire RE, Singer Y (2000) BoosTexter: a boosting-based system for text categorization. Mach Learn 39(2/3):135–168
Gardner MW, Dorling SJA (1998) Artificial neural networks (the multilayer perceptron). A rev appl atmo sci. 32:2627–2636
Chen, T. and C. Guestrin, 2016 XGBoost, in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 785–794.
Rodríguez-Pérez R, JBJC. Design, 2020 Interpretation of machine learning models using shapley values: application to compound potency and multi-target activity predictions. 34(10): 1013–1026.
Sullivan L.M., J.M. Massaro, and R.B.D.A.J.S.i.M. Sr., 2010 Presentation of multivariate data for clinical use: The Framingham Study risk score functions. 23(10): 1631–1660.
Woodward M (2013) Epidemiology: study design and data analysis. CRC press
Fang Y, Pei Z, Chen H, Wang R, Feng M, Wei L, Li J, Zhang H, Wang S (2021) Diagnostic value of knosp grade and modified knosp grade for cavernous sinus invasion in pituitary adenomas: a systematic review and meta-analysis. Pituitary 24(3):457–464
Athanasios F et al (2018) Recurrence of Silent Corticotroph Adenomas After Primary Treatment: A Systematic Review. Meta-Analysis 4:4
Bradley KJ, Wass JAH, Turner HEJCE (2010) Non-functioning pituitary adenomas with positive immunoreactivity for ACTH behave more aggressively than ACTH immunonegative tumours but do not recur more frequently. Clinical Endocrinol 58(1):665
Li H, Zhao Q, Zhang Y, Sai K, Xu L, Mou Y et al (2021) Image-driven classification of functioning and nonfunctioning pituitary adenoma by deep convolutional neural networks. Computat Struct Biotech J 19:3077–3086
Kasuki L et al (2019) Accuracy of microcystic aspect on T2 eighted MRI for the diagnosis of silent corticotroph adenomas. Clin Endocrinol 92(2):676
Funding
This work was supported by the National Key R&D Program of China (2021YFE0114300); CAMS Innovation Fund for Medical Sciences (CIFMS 2021-I2M-1–003); Beijing Municipal Natural Science Foundation (M22013); Key-Area Research and Development Program of Guangdong Province (2021B0101420005); National High-Level Hospital Clinical Research Funding (2022-PUMCH-C-012).
Author information
Authors and Affiliations
Contributions
HW and WZ contributed equally to the present study. MF initiated the study. FF collected MR images, HW, WZ, and MF performed segmentation. YY, KD, LL, and XB helped collect neurosurgery data and endocrinology data. HW and YF established the model. SL, SJ, and RW wrote the draft. All authors revised the manuscript critically. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflicts of interest.
Ethical approval
This retrospective study was approved by the ethical review committee of the Peking Union Medical College Hospital.
Informed consent
The requirement for informed consent was waived because of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Chang, J., Zhang, W. et al. Radiomics model and clinical scale for the preoperative diagnosis of silent corticotroph adenomas. J Endocrinol Invest 46, 1843–1854 (2023). https://doi.org/10.1007/s40618-023-02042-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02042-2