Abstract
Purpose
To assess the causal effects of serum testosterone and sex hormone-binding globulin levels on brain volumetric measurements in women and men.
Methods
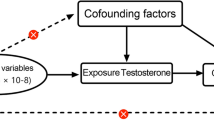
We performed a sex-stratified two-sample Mendelian randomization study using the random-effects inverse variance-weighted method as the primary analysis method. Sex-specific genetic instruments were obtained from a study with up to 194,453 men and 230,454 women. For testosterone, variants with dominant effects on both total and bioavailable testosterone but no aggregate effect on sex hormone-binding globulin were used as the main genetic instruments. Sex-specific summary-level data for magnetic resonance imaging brain volumetric measurements were obtained from a study with 11,624 women and 10,514 men.
Results
Analyses showed per standard deviation (approximately 3.7 nmol/L) higher testosterone levels in men were suggestively associated with larger gray matter volume (beta = 0.208, 95% confidence interval = 0.067 to 0.349, p = 0.004). The association remained in sensitivity analyses and multivariable analyses. Further analyses showed the effect was mainly act on peripheral cortical gray matter, but not on subcortical gray matter. Testosterone in men was not associated with hippocampal volume. Testosterone in women and sex hormone binding globulin in both sexes had no effect on all outcomes.
Conclusion
Our findings overall support previous evidence that testosterone might have neuroprotective properties in elderly men. Future larger trials with long duration of intervention are warranted to assess the efficacy of testosterone for elderly men with cognitive impairment, especially in those with hypoandrogenism.




Similar content being viewed by others
Data availability statement
The data supporting the findings of this study are available in the article’s supplementary files, and in the studies cited in this article.
Abbreviations
- CI:
-
Confidence interval
- GWAS:
-
Genome wide association study
- MR:
-
Mendelian randomization
- MR-PRESSO:
-
MR pleiotropy residual sum and outlier
- IVW:
-
Inverse variance-weighted
- SNP:
-
Single nucleotide polymorphism
References
Royle NA, Booth T, Valdes Hernandez MC, Penke L, Murray C, Gow AJ et al (2013) Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol Aging 34(12):2726–2733. https://doi.org/10.1016/j.neurobiolaging.2013.05.015
van Loenhoud AC, Groot C, Vogel JW, van der Flier WM, Ossenkoppele R (2018) Is intracranial volume a suitable proxy for brain reserve? Alzheimers Res Ther 10(1):91. https://doi.org/10.1186/s13195-018-0408-5
Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM et al (1995) Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. AJNR Am J Neuroradiol 16(2):241–251
Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F et al (2008) Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology 248(2):590–598. https://doi.org/10.1148/radiol.2482070938
Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC et al (2009) Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 72(11):999–1007. https://doi.org/10.1212/01.wnl.0000344568.09360.31
Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C et al (2011) Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging 32(4):572–580. https://doi.org/10.1016/j.neurobiolaging.2009.04.011
Fanaei H, Karimian SM, Sadeghipour HR, Hassanzade G, Kasaeian A, Attari F et al (2014) Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Brain Res 1558:74–83. https://doi.org/10.1016/j.brainres.2014.02.028
Elbejjani M, Schreiner PJ, Siscovick DS, Sidney S, Lewis CE, Bryan NR et al (2017) Sex hormones and brain volumes in a longitudinal study of middle-aged men in the CARDIA study. Brain Behav 7(10):e00765. https://doi.org/10.1002/brb3.765
Lessov-Schlaggar CN, Reed T, Swan GE, Krasnow RE, DeCarli C, Marcus R et al (2005) Association of sex steroid hormones with brain morphology and cognition in healthy elderly men. Neurology 65(10):1591–1596. https://doi.org/10.1212/01.wnl.0000184512.08249.48
Heany SJ, van Honk J, Stein DJ, Brooks SJ (2016) A quantitative and qualitative review of the effects of testosterone on the function and structure of the human social-emotional brain. Metab Brain Dis 31(1):157–167. https://doi.org/10.1007/s11011-015-9692-y
Neufang S, Specht K, Hausmann M, Gunturkun O, Herpertz-Dahlmann B, Fink GR et al (2009) Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex 19(2):464–473. https://doi.org/10.1093/cercor/bhn100
Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R (2010) Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage 49(2):1205–1212. https://doi.org/10.1016/j.neuroimage.2009.09.046
Yuan C, Jian Z, Jin X (2022) Chronotype and insomnia may affect the testosterone levels with a sexual difference: a Mendelian randomization. J Endocrinol Invest. https://doi.org/10.1007/s40618-022-01890-8
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM et al (2019) Guidelines for performing Mendelian randomization investigations. Wellcome Open Res 4:186. https://doi.org/10.12688/wellcomeopenres.15555.2
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D et al (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife. https://doi.org/10.7554/eLife.34408
Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A et al (2020) Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 26(2):252–258. https://doi.org/10.1038/s41591-020-0751-5
Vermeulen A, Verdonck L, Kaufman JM (1999) A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84(10):3666–3672. https://doi.org/10.1210/jcem.84.10.6079
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F et al (2019) Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51(2):237–244. https://doi.org/10.1038/s41588-018-0307-5
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN et al (2018) Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet 27(20):3641–3649. https://doi.org/10.1093/hmg/ddy271
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW et al (2018) Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet 50(11):1505–1513. https://doi.org/10.1038/s41588-018-0241-6
Zheng B-K, Niu P-P (2022) Higher coffee consumption is associated with reduced cerebral gray matter volume: a Mendelian randomization study. Front Nutr. https://doi.org/10.3389/fnut.2022.850004
Cole JB, Florez JC, Hirschhorn JN (2020) Comprehensive genomic analysis of dietary habits in UK Biobank identifies hundreds of genetic associations. Nat Commun 11(1):1467. https://doi.org/10.1038/s41467-020-15193-0
Georgakis MK, Harshfield EL, Malik R, Franceschini N, Langenberg C, Wareham NJ et al (2021) Diabetes mellitus, glycemic traits, and cerebrovascular disease: a Mendelian randomization study. Neurology 96(13):e1732–e1742. https://doi.org/10.1212/WNL.0000000000011555
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM et al (2015) A global reference for human genetic variation. Nature 526(7571):68–74. https://doi.org/10.1038/nature15393
Burgess S, Davies NM, Thompson SG (2016) Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 40(7):597–608. https://doi.org/10.1002/gepi.21998
Smith SM, Douaud G, Chen W, Hanayik T, Alfaro-Almagro F, Sharp K et al (2021) An expanded set of genome-wide association studies of brain imaging phenotypes in UK Biobank. Nat Neurosci 24(5):737–745. https://doi.org/10.1038/s41593-021-00826-4
Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JLR, Griffanti L, Douaud G et al (2018) Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166:400–424. https://doi.org/10.1016/j.neuroimage.2017.10.034
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR (2016) Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol 45(6):1961–1974. https://doi.org/10.1093/ije/dyw220
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698. https://doi.org/10.1038/s41588-018-0099-7
Hemani G, Tilling K, Davey SG (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 13(11):e1007081. https://doi.org/10.1371/journal.pgen.1007081
Xue A, Wu Y, Zhu Z, Zhang F, Kemper KE, Zheng Z et al (2018) Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun 9(1):2941. https://doi.org/10.1038/s41467-018-04951-w
Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H et al (2018) Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet 50(10):1412–1425. https://doi.org/10.1038/s41588-018-0205-x
Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G et al (2020) Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 17(3):e1003062. https://doi.org/10.1371/journal.pmed.1003062
Pan-UKB team. https://pan.ukbb.broadinstitute.org. 2020. Accessed date: March 02 2022.
Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT et al (2019) Biological and clinical insights from genetics of insomnia symptoms. Nat Genet 51(3):387–393. https://doi.org/10.1038/s41588-019-0361-7
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M et al (2019) Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22(3):343–352. https://doi.org/10.1038/s41593-018-0326-7
Bhasin S (2021) Testosterone replacement in aging men: an evidence-based patient-centric perspective. J Clin Invest. https://doi.org/10.1172/JCI146607
Zhao X, Liang S, Wang N, Hong T, Sambou ML, Fan J et al (2021) Sex-specific associations of testosterone and genetic factors with health span. Front Endocrinol (Lausanne) 12:773464. https://doi.org/10.3389/fendo.2021.773464
Marriott RJ, Murray K, Flicker L, Hankey GJ, Matsumoto AM, Dwivedi G et al (2022) Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: the UK Biobank prospective cohort study. Alzheimers Dement. https://doi.org/10.1002/alz.12529
Dong X, Jiang H, Li S, Zhang D (2021) Low serum testosterone concentrations are associated with poor cognitive performance in older men but not women. Front Aging Neurosci 13:712237. https://doi.org/10.3389/fnagi.2021.712237
Lv W, Du N, Liu Y, Fan X, Wang Y, Jia X et al (2016) Low testosterone level and risk of Alzheimer’s disease in the elderly men: a systematic review and meta-analysis. Mol Neurobiol 53(4):2679–2684. https://doi.org/10.1007/s12035-015-9315-y
Corona G, Guaraldi F, Rastrelli G, Sforza A, Maggi M (2021) Testosterone deficiency and risk of cognitive disorders in aging males. World J Mens Health 39(1):9–18. https://doi.org/10.5534/wjmh.200017
Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA et al (2017) Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 317(7):717–727. https://doi.org/10.1001/jama.2016.21044
Mohammadi-Shemirani P, Chong M, Pigeyre M, Morton RW, Gerstein HC, Pare G (2020) Effects of lifelong testosterone exposure on health and disease using Mendelian randomization. Elife. https://doi.org/10.7554/eLife.58914
Jayadevappa R, Chhatre S, Malkowicz SB, Parikh RB, Guzzo T, Wein AJ (2019) Association between androgen deprivation therapy use and diagnosis of dementia in men with prostate cancer. JAMA Netw Open 2(7):e196562. https://doi.org/10.1001/jamanetworkopen.2019.6562
Herting MM, Sowell ER (2017) Puberty and structural brain development in humans. Front Neuroendocrinol 44:122–137. https://doi.org/10.1016/j.yfrne.2016.12.003
Wierenga LM, Bos MGN, Schreuders E, Vd Kamp F, Peper JS, Tamnes CK et al (2018) Unraveling age, puberty and testosterone effects on subcortical brain development across adolescence. Psychoneuroendocrinology 91:105–114. https://doi.org/10.1016/j.psyneuen.2018.02.034
Pletzer B (2019) Sex hormones and gender role relate to gray matter volumes in sexually dimorphic brain areas. Front Neurosci 13:592. https://doi.org/10.3389/fnins.2019.00592
Lee JH, Byun MS, Yi D, Choe YM, Choi HJ, Baek H et al (2017) Sex-specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol Aging 58:34–40. https://doi.org/10.1016/j.neurobiolaging.2017.06.005
Savic I (2014) Asymmetry of cerebral gray and white matter and structural volumes in relation to sex hormones and chromosomes. Front Neurosci 8:329. https://doi.org/10.3389/fnins.2014.00329
Wang D, Han L, Xi C, Xu Y, Lai J, Lu S et al (2020) Interactive effects of gender and sexual orientation on cortical thickness, surface area and gray matter volume: a structural brain MRI study. Quant Imaging Med Surg 10(4):835–846. https://doi.org/10.21037/qims.2020.03.07
Suchy-Dicey A, Su Y, Buchwald DS, Manson SM, Reiman EM (2022) Volume atrophy in medial temporal cortex and verbal memory scores in American Indians: data from the strong heart study. Alzheimers Dement. https://doi.org/10.1002/alz.12889
Katsumi Y, Putcha D, Eckbo R, Wong B, Quimby M, McGinnis S et al (2022) Anterior dorsal attention network tau drives visual attention deficits in posterior cortical atrophy. Brain. https://doi.org/10.1093/brain/awac245
Bianchi VE (2022) Impact of testosterone on Alzheimer’s disease. World J Mens Health. https://doi.org/10.5534/wjmh.210175
Yan XS, Yang ZJ, Jia JX, Song W, Fang X, Cai ZP et al (2019) Protective mechanism of testosterone on cognitive impairment in a rat model of Alzheimer’s disease. Neural Regen Res 14(4):649–657. https://doi.org/10.4103/1673-5374.245477
Lau CF, Ho YS, Hung CH, Wuwongse S, Poon CH, Chiu K et al (2014) Protective effects of testosterone on presynaptic terminals against oligomeric beta-amyloid peptide in primary culture of hippocampal neurons. Biomed Res Int 2014:103906. https://doi.org/10.1155/2014/103906
Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S et al (2012) Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PLoS ONE 7(1):e29598. https://doi.org/10.1371/journal.pone.0029598
Wang X, Lv Z, Wu Q, Liu H, Gu Y, Ye T (2021) Lower plasma total testosterone levels were associated with steeper decline in brain glucose metabolism in non-demented older men. Front Aging Neurosci 13:592845. https://doi.org/10.3389/fnagi.2021.592845
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study and the UK Biobank. We also want to acknowledge the participants and investigators of all other studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
P-PN: conceptualization, formal analysis, methodology, writing original draft. XW: data curation, writing original draft. Y-MX: project administration, Supervision, reviewing and editing manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval and informed consent
Ethical approve and informed consent were obtained in the original studies. They were waived for the present study because only summary-level data were used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, PP., Wang, X. & Xu, YM. Causal effects of serum testosterone levels on brain volume: a sex-stratified Mendelian randomization study. J Endocrinol Invest 46, 1787–1798 (2023). https://doi.org/10.1007/s40618-023-02028-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-023-02028-0