Abstract
Objective
Although several studies have reported that testosterone may protect against Alzheimer’s disease, no evidence of a causal relationship has been demonstrated.
Methods
A Mendelian randomization (MR) study was performed to determine the causal role of testosterone in Alzheimer’s disease. The study utilized public databases obtained from separately published genome-wide associationstudies (GWAS). Single-nucleotide polymorphisms (SNPs) for testosterone were extracted from the most recent and largest published GWAS meta-analysis (178,782 participants), and SNPs for Alzheimer’s disease were extracted from UK Biobank (954 AD cases and 487,331 controls). The odds ratio (OR) of the inverse variance weighting (IVW) approach was the primary outcome, and the weighted median and MR Egger regression were used for sensitivity analysis.
Results
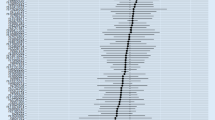
Through IVW, we observed a causal association between genetically predicted testosterone and the risk of Alzheimer’s disease, with an OR of 0.99 (95% confidence interval [CI] = 0.998–0.999, p = 0.047). In the sensitivity analyses, the weighted median regression showed directionally similar estimates (OR = 0.99, 95% CI = 0.998–0.999, p = 0.048). The MR Egger regression showed similar estimates (OR = 0.99, 95% CI = 0.998–1.00, p = 0.35), but with lower precision. Funnel plots, MR Egger intercepts, and Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) analysis indicated the absence of directional pleiotropy effects.
Conclusion
In conclusion, our MR study provides evidence of a causal relationship between testosterone levels and Alzheimer’s disease; however, this relationship must be validated in future studies with larger sample sizes. Early testosterone monitoring may enable the prevention of Alzheimer’s and related diseases.






Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
References
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 10:73–82. https://doi.org/10.1080/01616412.2016.1251711
Feigin VL, Nichols E, Alam T et al (2019) GBD 2016 Neurology collaborators. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global burden of disease study 2016. Lancet Neurol 18(5):459–480
United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons (2020) (ST/ESA/SER.A/451). Available online: https://www.un.org/development/ desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd-2020_world_population_ageing_highlights.pdf. (Accessed on 4 April 2021)
Prince M, Albanese E, Guerchet M et al (2014) World Alzheimer report 2014 dementia and risk reduction an analysis of protective and modifiable factors. Alzheimer’s Disease International (ADI), September 2014. Available online: https://www.researchgate.net/publication/266088301
Tobore TO (2022) On the etiopathogenesis and pathophysiology of Alzheimer’s disease: a comprehensive theoretical review. J Alzheimers Dis 2:417–437. https://doi.org/10.3233/JAD-181052
Tatulian SA (2022) Challenges and hopes for Alzheimer’s disease. Drug Discov Today 27(4):1027–1043. https://doi.org/10.1016/j.drudis.2022.01.016
Barron AM, Pike CJ (1970) Sex hormones, aging, and Alzheimer’s disease. Front Biosci (Elite Ed) 3:976–97. https://doi.org/10.2741/E434
Lei Y, Renyuan Z (2018) Effects of androgens on the amyloid-β protein in Alzheimer’s disease. Endocrinology 159(12):3885–3894. https://doi.org/10.1210/en.2018-00660
Pike CJ (2001) Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res 1:160–5. https://doi.org/10.1016/s0006-8993(01)03024-4
Yan XS, Yang ZJ, Jia JX et al (2019) Protective mechanism of testosterone on cognitive impairment in a rat model of Alzheimer’s disease. Neural Regen Res 14(4):649–657. https://doi.org/10.4103/1673-5374.245477
Sun M, Cole AP, Hanna N et al (2018) Cognitive impairment in men with prostate cancer treated with androgen deprivation therapy: a systematic review and meta-analysis. J Urol 199(6):1417–1425. https://doi.org/10.1016/j.juro.2017.11.136
Deka R, Simpson DR, Bryant AK et al (2018) Association of androgen deprivation therapy with dementia in men with prostate cancer who receive definitive radiation therapy. JAMA Oncol 4(11):1616–1617. https://doi.org/10.1001/jamaoncol.2018.4423
Buskbjerg CR, Gravholt CH, Dalby HR, Amidi A, Zachariae R (2019) Testosterone supplementation and cognitive functioning in men-a systematic review and meta-analysis. J Endocr Soc 3(8):1465–1484. https://doi.org/10.1210/js.2019-00119
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA 19:1925–1926. https://doi.org/10.1001/jama.2017.17219
Davies NM, Holmes MV, Davey Smith G (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. https://doi.org/10.1136/bmj.k601
Richmond RC, Hemani G, Tilling K et al (2016) Challenges and novel approaches for investigating molecular mediation. Hum Mol Genet 25(R2):R149–R156. https://doi.org/10.1093/hmg/ddw197
Tan JS, Liu NN et al (2021) Genetically predicted obesity and risk of deep vein thrombosis. Thromb Res 207:16–24. https://doi.org/10.1016/j.thromres.2021.08.026
Ruth KS, Day FR, Tyrrell J et al (2020) Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med 26(2):252–258. https://doi.org/10.1038/s41591-020-0751-5
VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P (2014) Methodological challenges in mendelian randomization. Epidemiology 25(3):427–435. https://doi.org/10.1097/EDE.0000000000000081
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37(7):658–665. https://doi.org/10.1002/gepi.21758
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314. https://doi.org/10.1002/gepi.21965
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525. https://doi.org/10.1093/ije/dyv080
Zhang Y, Liu Z, Choudhury T, Cornelis MC, Liu W (2021) Habitual coffee intake and risk for nonalcoholic fatty liver disease: a two-sample Mendelian randomization study. Eur J Nutr 60(4):1761–1767. https://doi.org/10.1007/s00394-020-02369-z
Broadbent JR, Foley CN, Grant AJ, Mason AM, Staley JR, Burgess S (2020) MendelianRandomization v0.5.0: updates to an R package for performing Mendelian randomization analyses using summarized data. Wellcome Open Res. https://doi.org/10.12688/wellcomeopenres.16374.2
Moffat SD, Zonderman AB, Metter EJ et al (2004) Free testosterone and risk for Alzheimer disease in older men. Neurology 2:188–93. https://doi.org/10.1212/wnl.62.2.188
Kische H, Gross S, Wallaschofski H et al (2017) 2017 Associations of androgens with depressive symptoms and cognitive status in the general population. PLoS One 12(5):e0177272. https://doi.org/10.1371/journal.pone.0177272. (Published 2017 May 12)
Gillett MJ, Martins RN, Clarnette RM, Chubb SA, Bruce DG, Yeap BB (2003) Relationship between testosterone, sex hormone binding globulin and plasma amyloid beta peptide 40 in older men with subjective memory loss or dementia. J Alzheimers Dis 5(4):267–269. https://doi.org/10.3233/jad-2003-5401
Feldman HA, Longcope C, Derby CA et al (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2:589–98. https://doi.org/10.1210/jcem.87.2.8201
Rosario ER, Chang L, Stanczyk FZ et al (2004) Age-related testosterone depletion and the development of Alzheimer disease. JAMA 12:1431–2. https://doi.org/10.1001/jama.292.12.1431-b
Lee JH, Byun MS, Yi D et al (2017) Sex-specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol Aging 58:34–40. https://doi.org/10.1016/j.neurobiolaging.2017.06.005
Yao PL, Zhuo S, Mei H et al (2017) Androgen alleviates neurotoxicity of β-amyloid peptide (Aβ) by promoting microglial clearance of Aβ and inhibiting microglial inflammatory response to Aβ. CNS Neurosci Ther 23(11):855–865. https://doi.org/10.1111/cns.12757
Huo DS, Sun JF, Zhang B et al (2016) Protective effects of testosterone on cognitive dysfunction in Alzheimer’s disease model rats induced by oligomeric beta amyloid peptide 1–42. J Toxicol Environ Health A 79(19):856–863. https://doi.org/10.1080/15287394.2016.1193114
Resnick SM, Matsumoto AM, Stephens-Shields AJ et al (2017) Testosterone treatment and cognitive function in older men with low testosterone and age-associated memory impairment. JAMA 317(7):717–727. https://doi.org/10.1001/jama.2016.21044
Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR et al (2008) Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 299(1):39–52. https://doi.org/10.1001/jama.2007.51. (Published correction appears in JAMA. 2008 Feb 13;299(6):634)
Vaughan C, Goldstein FC, Tenover JL (2007) Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl 28(6):875–882. https://doi.org/10.2164/jandrol.107.002931
Cherrier MM, Matsumoto AM, Amory JK et al (2007) Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology 32(1):72–79. https://doi.org/10.1016/j.psyneuen.2006.10.008
Goodenough S, Engert S, Behl C (2000) Testosterone stimulates rapid secretory amyloid precursor protein release from rat hypothalamic cells via the activation of the mitogen-activated protein kinase pathway. Neurosci Lett 296(1):49–52. https://doi.org/10.1016/s0304-3940(00)01622-0
Vassar R, Kuhn PH, Haass C et al (2014) Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. J Neurochem 130(1):4–28. https://doi.org/10.1111/jnc.12715
McAllister C, Long J, Bowers A et al (2010) Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents Alzheimer-like pathology and cognitive impairment. J Neurosci 30(21):7326–7334. https://doi.org/10.1523/JNEUROSCI.1180-10.2010
Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A (2004) Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci 24(23):5315–5321. https://doi.org/10.1523/JNEUROSCI.0913-04.2004
Jia JX, Cui CL, Yan XS et al (2016) Effects of testosterone on synaptic plasticity mediated by androgen receptors in male SAMP8 mice. J Toxicol Environ Health A 79(19):849–855. https://doi.org/10.1080/15287394.2016.1193113
Acknowledgements
We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.The authors would like to thank all the genetics consortiums for making the GWAS summary data publicly available.
Funding
There was no funding for this research.
Author information
Authors and Affiliations
Contributions
XQ and ZJ designed the study. XQ, ZY, and SH conducted research. XQ and Zhang J analyzed the data. XQ, SZ, JJ, and SH wrote this paper. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Shen, H., Zhu, Y. et al. Causal effects of genetically predicted testosterone on Alzheimer’s disease: a two-sample mendelian randomization study. Acta Neurol Belg 124, 591–601 (2024). https://doi.org/10.1007/s13760-023-02426-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02426-4