Abstract
Purpose
Acromegaly and neuroendocrine tumors are rare diseases that, under certain conditions, can be treated with somatostatin analogs. The aim was to determine the prescription patterns of somatostatin analogs in a group of patients with acromegaly and neuroendocrine tumors affiliated with the Colombian Health System.
Methods
A retrospective study. A cohort of patients from a drug dispensing database that collected all prescriptions of long-acting somatostatin analogs (octreotide, lanreotide, pasireotide). Sociodemographic variables, clinical variables (diagnosis and comorbidities) and pharmacological therapy variables (dose, changes, persistence of use, comedications) were considered.
Results
A total of 213 patients were identified, including 139 (65.3%) with acromegaly and 74 (34.7%) with neuroendocrine tumors. There was a predominance of women (58.7%) and a mean age of 59.7 ± 14.5 years. The most commonly used medications were lanreotide autogel (n = 107; 50.2%), octreotide LAR (n = 102; 47.9%) and pasireotide LAR (n = 4; 1.9%). During follow-up, 11.3% of patients experienced modifications of therapy, with a mean duration from the beginning of treatment to the change in medication of 25 ± 15.9 months. A total of 48.9% of the patients with acromegaly and 87.1% of individuals with neuroendocrine tumors received maximum approved doses of the drug.
Conclusion
Patients with acromegaly and neuroendocrine tumors in Colombia are mainly women and are most frequently treated with lanreotide autogel for acromegaly and with octreotide LAR for neuroendocrine tumors. In addition, a high proportion are managed with maximum doses of long-acting somatostatin analogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a relatively rare disease with an estimated global prevalence of 40–50 cases per million inhabitants [1, 2]. It leads to alterations in patients’ quality of life and early mortality, mainly due to cardiovascular causes, and consequently generates high costs related to both medical and surgical treatments [3, 4]. The management of these patients is complex and aims to control symptoms, normalize hormone excess, and reduce tumor volume [4]. First-line treatment usually involves trans-sphenoidal adenectomy (in patients with completely resectable tumors or compromised vision), but in patients who are not eligible candidates for this procedure, those with unsuccessful pituitary surgery and those who reject the surgery [5], somatostatin analogs, such as octreotide and lanreotide, are recommended [4, 6,7,8]. These drugs are also used as first-line therapy in patients with un-resectable neuroendocrine tumors, which have a prevalence of less than 1%, although their incidence has increased in recent years [9]. Somatostatin analogs are also used for symptom control of metastatic carcinoid tumors, such as flushing, wheezing, telangiectasias and diarrhea [10].
Somatostatin analogs preferentially bind to somatostatin receptors SST2 and SST5, suppressing growth hormone (GH) secretion [11, 12], effectively controlling the biochemical parameters of acromegaly in 60 to 80% of patients (normal values of insulin-like growth factor 1 (IGF-1) and GH < 1 μg/L) [5]. In neuroendocrine tumors, they can lead to anti-proliferative effects by decreasing cell cycles and activating the apoptosis of cells expressing somatostatin receptors, leading to a decrease in tumor angiogenesis and growth factors expression and a reduction in the secretion of hormonal peptides [13, 14], and reduction in skin flushing and diarrhea in up to 88% of patients, as well [15]. Despite being a fairly effective therapy, the costs of this approach are significant, and in some cases and some countries, it is difficult or impossible to access to these medications, leading doctors to use alternative treatments [3, 16, 17].
In a report of patients in Colombia with acromegaly and neuroendocrine tumors (between 2011 and 2015), the use of long-acting somatostatin analogs was described. Octreotide was the most frequently used (in 56.1% of the cases), followed by lanreotide (43.9%). In addition, 4.5% of patients changed from one medication to another [17]. Furthermore, in 2013, pasireotide LAR became available in the public health system formulary. It is another somatostatin analog also administered every 28 days and approved for patients with acromegaly [18, 19].
Since acromegaly is a condition hidden by many comorbid disorders, its diagnosis is usually delayed for several years leading to increased mortality versus the general population [20, 21]. Similarly, gastroenteropancreatic neuroendocrine tumors are likely to be diagnosed in advanced stage due to many gastrointestinal disorders masking the neuroendocrine tumor and increasing the complexity and the cost of the treatment. [9, 10, 22, 23]. Therefore, the management of these conditions in the real-life setting is a topic of interest. The Colombian Health System offers universal coverage to the entire population through two regimes: the contributory scheme, which is paid by workers and employers, and another scheme that is subsidized by the state. It also has a benefit plan (PBS) that includes some medications used for the treatment of acromegaly and neuroendocrine tumors. Thus, it was proposed to determine the prescription and use patterns of somatostatin analogs in a group of patients with acromegaly and neuroendocrine tumors affiliated with the Colombian Health System between 2015 and 2020.
Methods
A retrospective study of a cohort of patients was conducted aiming to describe the prescription pattern of long-acting somatostatin analogs (octreotide LAR, lanreotide autogel and pasireotide LAR) and their dispensation trend over time from 2015 to 2020. From a population of 6.5 million people affiliated with the contributory and subsidized regimes of the Colombian Health System of different health insurance entities, patients with prescriptions for somatostatin analogs were identified, and a monthly follow-up was performed with the objective of evaluating changes in therapy.
The study database, validated and reviewed by a pharmacologist, was designed to allow the collection of groups of patient variables during the observation period. These variables are described below:
-
(1)
Sociodemographic variables: Age, sex and affiliation regime (contributory or subsidized).
-
(2)
Clinical variables: Clinical diagnosis identified in the prescription (differentiating between patients with acromegaly or neuroendocrine tumor), modifications (dose or medication) and persistence of treatment use, determined by the number of dispensations of drugs (persistence during the first 12 months of use).
-
(3)
Use of somatostatin analogs (patterns and dispensation trend): Number of patients with a monthly prescription during the follow-up period (trend), specialty of the prescribing physician and the prescribed dose. To quantify the amount dispensed, the defined daily dose (DDD) recommended by the World Health Organization (WHO) as the international standard for pharmaco-epidemiological studies was used as the technical unit of measurement.
-
(4)
The following comorbidities and their respective medications were considered: (a) diabetes mellitus; (b) Parkinson's disease; (c) HIV-AIDS; (d) depressive disorder; (e) chronic obstructive pulmonary disease; (f) dyslipidemia; (g) hypothyroidism; (h) ischemic heart disease; (i) arterial hypertension, (j) hyperprolactinemia, (k) cardiac arrhythmia, and (l) rheumatoid arthritis.
For the analysis of the data, the statistical package SPSS Statistics, version 26.0 (IBM, USA) for Windows was used. Means with standard deviation and frequency with proportions were calculated. The sociodemographic and usage variables, including changes in therapy and co-medication, were reported for the total population of somatostatin analog users (patterns and trend of use). The persistence of use (number of months in which treatment was dispensed during the first year of follow-up), the total duration of use and the changes in therapy throughout the follow-up period, including the time (in months) until the first change in therapy, were identified.
DDD for the study molecules were the following: 3 mg/day for lanreotide (90 mg/month), 0.7 mg/day for octreotide (21 mg/month) and 1.2 mg/day for pasireotide (36 mg/month), according to the Anatomical Therapeutic Chemical (ATC) classification of the WHO. The ratio of the average dose administered to the patients to the monthly DDD (average dose/DDD) was calculated. Similarly, the proportions of patients with maximum doses and with doses higher than the maximum were calculated for each medication. The maximum doses were octreotide 30 mg/month, lanreotide 120 mg/month and pasireotide 60 mg/month.
The protocol was approved by the Bioethics Committee of the Universidad Tecnológica de Pereira in the category of risk-free research according to resolution 8430/1993 of the Ministry of Health of Colombia, and the principles established by the Declaration of Helsinki were respected (Endorsement Code: 20–150,321).
Results
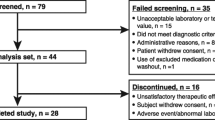
A total of 213 patients who used long-acting somatostatin analogs were included in the analysis. Of these, 139 were diagnosed with acromegaly (65.3%), and 74 were diagnosed with neuroendocrine tumors (34.7%). There was a predominance of females (n = 125; 58.7%) and an average age of 59.7 ± 14.5 years for the total included population. A total of 72.3% (n = 154) participated in the contributory regime of the health system. Table 1 shows the sociodemographic characteristics, comorbidities and concomitant therapies of each group of patients.
The general frequencies of use of the medications were as follows: lanreotide autogel (n = 107; 50.2%), octreotide LAR (n = 102; 47.9%) and pasireotide LAR (n = 4; 1.9%). Among the patients with acromegaly, the most commonly used complementary therapy was cabergoline (n = 48; 34.5%), while among those with neuroendocrine tumors, it was everolimus (n = 17; 23.0%). Table 2 shows the patterns of somatostatin analog use according to the disease.
Among the patients with acromegaly, 19 of those who used octreotide LAR (33.3%), 28 of those who used lanreotide autogel (35.9%) and one patient who used pasireotide LAR (25.0%) were treated concomitantly with cabergoline during the study period. Nine (20.0%) patients with neuroendocrine tumors who were treated with octreotide LAR and eight (27.6%) of those who used lanreotide autogel were concomitantly treated with everolimus. Of the patients with neuroendocrine tumors, only one who was treated with octreotide LAR (2.2%) and one who was treated with lanreotide autogel (3.4%) used sunitinib.
Among the patients with neuroendocrine tumors, the mean dose of octreotide LAR was higher than the maximum approved and recommended dose of 30 mg, while the mean dose of lanreotide autogel was lower than the maximum approved and recommended dose of 120 mg, as shown in Table 2, which presents the ratio of the mean dose to the maximum dose of the drugs.
During the follow-up period, 24 patients underwent treatment modifications (11.3%), with a mean duration of 25 ± 15.9 months from the beginning of treatment until the change in therapy. Modifications were more frequently observed in patients with acromegaly treated with octreotide LAR (14% switched to lanreotide autogel). Among those with neuroendocrine tumors, there were more patients who switched from octreotide to lanreotide (8.8%) than those who switched from lanreotide to octreotide (3.5%). Table 2 also shows the mean number of dispensations in the first year of use (persistence) and Table 3 shows the changes in the studied drugs according to the diagnosis.
Discussion
From the results obtained, it was possible to identify patients diagnosed with acromegaly and neuroendocrine tumors who were managed with long-acting somatostatin analogs during the five years of follow-up and to determine sociodemographic variables, comorbidities, concomitant therapies and treatment patterns associated with these medications. This information provides greater knowledge about pharmacological therapy based on real-life data that can be useful to improve access and care programs [2, 24].
The literature reports that most acromegaly patients are diagnosed at 40–45 years old [20]. The mean age in this study was 58 years, a fact that may be explained by the later diagnosis of this condition in Colombia than in developed countries and because patients who use long-acting somatostatin analogs usually have an evolution of the disease longer than 10 years (time between symptoms onset and the final diagnosis) [25]. In addition, there was a predominance of female patients among those diagnosed with acromegaly, similar to the findings of a previous study conducted in Colombia [17]. Among the patients diagnosed with neuroendocrine tumors, the mean age was similar to that reported in the literature, in which the median age at the diagnosis for a wide variety of gastroenteropancreatic neuroendocrine tumors is approximately 63 years, with slight variations according to the location of the tumor [23, 26, 27], in addition, it is clear to note that the frequency of neuroendocrine tumors treated with somatostatin analogs registered in the database is low, which can be explained because in many patients the ICD 10 diagnosis is not registered in the database or they use other therapies to control the disease.
The identification of comorbidities in the patients with acromegaly showed a high frequency of associated cardiovascular and metabolic disease [20], including a high prevalence of arterial hypertension, diabetes mellitus and dyslipidemia, conditions that increase the risk of cardiovascular events [28, 29]. This finding implies that the medical team should be aware of these possible comorbidities and actively search for them to provide timely treatment that can impact cardiovascular outcomes; additionally, acromegaly itself is associated with arterial hypertension, diabetes mellitus and cardiomyopathies [20, 29]. Furthermore, it should be noted that treatment with somatostatin analogs, especially pasireotide LAR, has been associated with hyperglycemia [30, 31]. Similarly, among the patients with neuroendocrine tumors, it was determined that main comorbidities were arterial hypertension and diabetes mellitus, as has been reported in epidemiological studies that identified these diagnoses and obesity as the main comorbidities of neuroendocrine tumors [10, 15]. More than 10% of the patients of this cohort were diagnosed with a depressive disorder. Such psychiatric comorbidity has been previously described and associated with the pathophysiology of neuroendocrine tumors [32] and may be a subject to explore in future studies and to be considered as part of holistic management of these patients. It is also noteworthy that the main concomitant medication in the management of acromegaly was cabergoline, a therapy that was only included in the PBS of the Colombian Health System until 2021, so patients required an extra approval to access this therapy, which explains why up to almost 10% received bromocriptine than if they were in the PBS and it was easier to access [33].
Analysis of long-acting somatostatin analogs used by patients with acromegaly showed a predominance of lanreotide autogel, which was used by more than half of the patients, followed by octreotide LAR and a small proportion of pasireotide LAR prescriptions in doses close to those recommended. However, approximately half of the users of octreotide LAR and lanreotide autogel were receiving the maximum doses, a situation similar to that reported in a similar study conducted in Colombia in 2017, which also found that octreotide LAR was used at higher doses than lanreotide autogel [17]. In addition, average doses that are higher than the maximum dose were identified in this study among patients with acromegaly being treated with octreotide LAR and among patients with neuroendocrine tumors; these high doses can lead to an increase in the associated treatment costs.
Another noteworthy point regarding somatostatin analogs is their persistence of use, because they are highly effective at normalizing GH concentrations in up to 70% of patients and IGF-1 in up to 80% [34, 35]. For many patients, prescription should be maintained for the long term or even indefinitely, since these drugs suppress GH secretion and their discontinuation can result in an adverse increase in GH [36]; an exception is in patients with a good response, in whom the doses can be spaced apart [37]. The present analysis identified that during the observation period, octreotide LAR and lanreotide autogel treatments were dispensed 5.7 and 7 times in the first year of treatment, respectively, for acromegaly patients; this suggests that the patients experienced interruptions in treatment, considering that these therapies should be administered on a monthly basis to achieve and maintain a biochemical and clinical control, at least at the beginning of treatment. This scenario was previously reported by [16] in a study of 1308 patients with acromegaly in the United States [16]. This situation could also impact patients’ quality of life, the control of the disease and its complications and the safety of the therapy [38]. These findings suggest the need for new studies that identify the factors associated with a lack of persistence in or adherence to the use of these drugs; examine the differences in effectiveness, real-world safety, impact on quality of life and control of comorbidities; and explore difficulties with access and delays in health systems’ administrative processes.
Regarding the use of somatostatin analogs among patients diagnosed with neuroendocrine tumors, octreotide LAR was used for a much longer time than lanreotide autogel. The most logical explanation for this finding is the continuity of therapy with octreotide LAR from monotherapy to combined therapy with everolimus or radionuclides once patients exhibit disease progression based on the evidence from the RADIANT-1, RADIANT-2 and NETTER-1 studies; however, these data require further investigation [37, 38].
Regarding the doses used by patients with neuroendocrine tumors, a higher mean monthly dose was identified for octreotide LAR compared to that of lanreotide autogel (32.4 mg vs. 117.9 mg, respectively) relative to the recommended doses (30 mg for octreotide and 120 mg for lanreotide). Additionally, twice as many patients received doses above the maximum for octreotide LAR than for lanreotide autogel. This finding of octreotide LAR prescriptions that exceeded the maximum dose of 30 mg every 28 days, particularly for patients with neuroendocrine tumors, coincides with the reports of similar studies in Europe and the United States. For example, the study by Fagnani F et al. evaluated the doses of lanreotide autogel and octreotide LAR recorded in the database of the national health system of France (which included 99% of the total population of the country) and reported the same finding as the present study, namely, that the proportion of patients with neuroendocrine tumors who were treated at doses higher than the maximum approved among those treated with octreotide LAR (11.2%, n = 100) doubled the proportion of patients treated with doses above the maximum recommended for lanreotide autogel (5.5%, n = 83)[11]. In the present study, these higher doses used in patients with neuroendocrine tumors was also noted when comparing the proportions of patients with maximum doses (above 80%) against those in the group of patients with acromegaly (below 50%).
A slightly higher number of dispensations for octreotide LAR than for lanreotide autogel was identified; however, this was still well below the expected number of dispensations (1 per month = 12 per year), a finding to be further investigated in future studies.
In determining the impact of this study, some important limitations should be taken into account, such as the small number of patients included, which is related to the low prevalence of acromegaly and neuroendocrine tumors, and the lack of such clinical data as the severity of the disease, the location and type of neuroendocrine tumors, the history of surgical management and the reasons for therapy change or discontinuation. Detailed information on the prescription was not available to accurately determine the dosing interval, thus the dose/month was calculated from monthly drug deliveries on usual dosing basis every 4 weeks. All this information could be useful to explain some of the findings related in the present study. For this same reason, conclusions regarding adherence and persistence may have limited comparability among patients.
Based on above findings, it can be concluded that patients with acromegaly and neuroendocrine tumors in Colombia are primarily women who receive lanreotide autogel more frequently for the treatment of acromegaly and octreotide LAR more frequently for neuroendocrine tumors. In addition, approximately half of patients with acromegaly and more than 80% of those with neuroendocrine tumors receive the maximum doses of the different somatostatin analogs, with a low proportion of switching between drugs and intermediate persistence of use throughout the first year of treatment. These results can be useful for physicians in the areas of neurosurgery, endocrinology, and oncology and for decision-makers, as well, to improve treatment adherence, medication use and the clinical outcomes in these patients.
Data availability
protocols.io. dx.doi.org/10.17504/protocols.io.n92ldz5xov5b/v1.
References
Chanson P, Salenave S, Kamenicky P, Cazabat L, Young J (2009) Pituitary tumours: acromegaly. Best Pract Res Clin Endocrinol Metab 23:555–574. https://doi.org/10.1016/j.beem.2009.05.010
Melmed S (2009) Acromegaly pathogenesis and treatment. J Clin Invest 119:3189–3202. https://doi.org/10.1172/JCI39375
Ben-Shlomo A, Sheppard MC, Stephens JM, Pulgar S, Melmed S (2011) Clinical, quality of life, and economic value of acromegaly disease control. Pituitary 14:284–294. https://doi.org/10.1007/s11102-011-0310-7
Capatina C, Wass JA (2015) 60 YEARS OF NEUROENDOCRINOLOGY: acromegaly. J Endocrinol 226:T141–T160. https://doi.org/10.1530/JOE-15-0109
Grasso LF, Pivonello R, Colao A (2012) Somatostatin analogs as a first-line treatment in acromegaly: when is it appropriate? Curr Opin Endocrinol Diabetes Obes 19:288–294. https://doi.org/10.1097/MED.0b013e328354af67
Barkan A, Bronstein MD, Bruno OD, Cob A, Espinosa-de-los-Monteros AL, Gadelha MR, Garavito G, Guitelman M, Mangupli R, Mercado M, Portocarrero L, Sheppard M (2010) Management of acromegaly in Latin America: expert panel recommendations. Pituitary 13:168–175. https://doi.org/10.1007/s11102-009-0206-y
Bronstein MD, Bruno OD, Abreu A, Mangupli R, Mercado M (2014) A practical approach to acromegaly management in Latin America. Pituitary 17:S30–S35. https://doi.org/10.1007/s11102-013-0531-z
Giustina A, Bronstein MD, Casanueva FF, Chanson P, Ghigo E, Ho KK, Klibanski A, Lamberts S, Trainer P, Melmed S (2011) Current management practices for acromegaly: an international survey. Pituitary 14:125–133. https://doi.org/10.1007/s11102-010-0269-9
Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342. https://doi.org/10.1001/jamaoncol.2017.0589
Darbà J, Marsà A (2019) Exploring the current status of neuroendocrine tumours: a population-based analysis of epidemiology, management and use of resources. BMC Cancer 19:1226. https://doi.org/10.1186/s12885-019-6412-8
Fagnani F, Feuilly M, Marteau F, de Zélicourt M, Kamenicky P, de Mestier L. Lanreotide autogel and octreotide LAR treatment patterns: results from a nationwide French retrospective study. 17th Annual ENETS Conference | 11–13 March 2020; Barcelona, Spain.2020. https://www.enets.org/lanreotide-autogel-and-octreotide-lar-treatment-patterns-results-from-a-nationwide-french-retrospective-study.html Accessed on Mar 1 2022.
Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C (2020) Somatostatin analogs in clinical practice: a Review. Int J Mol Sci 21:1682. https://doi.org/10.3390/ijms21051682
Nilsson O, Kölby L, Wängberg B, Wigander A, Billig H, William-Olsson L, Fjälling M, Forssell-Aronsson E, Ahlman H (1998) Comparative studies on the expression of somatostatin receptor subtypes, outcome of octreotide scintigraphy and response to octreotide treatment in patients with carcinoid tumours. Br J Cancer 77:632–637. https://doi.org/10.1038/bjc.1998.101
Theodoropoulou M, Stalla GK (2013) Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 34:228–252. https://doi.org/10.1016/j.yfrne.2013.07.005
Perez K, Chan J (2019) Treatment of gastroenteropancreatic neuroendocrine tumors. Surg Pathol Cli 12:1045–1053. https://doi.org/10.1016/j.path.2019.08.011
Gurel MH, Han Y, Stevens AL, Furtado A, Cox D (2017) Treatment adherence and persistence with long-acting somatostatin analog therapy for the treatment of acromegaly: a retrospective analysis. BMC Pharmacol Toxicol 18:22. https://doi.org/10.1186/s40360-017-0124-y
Machado-Alba JE, Machado-Duque ME (2017) Prescription patterns of long-acting somatostatin analogues. SAGE Open Med 5:2050312117694795. https://doi.org/10.1177/2050312117694795
Colao A, Bronstein MD, Brue T, De Marinis L, Fleseriu M, Guitelman M, Raverot G, Shimon I, Fleck J, Gupta P, Pedroncelli AM, Gadelha MR (2020) Pasireotide for acromegaly: long-term outcomes from an extension to the Phase III PAOLA study. Eur J Endocrinol 182:583. https://doi.org/10.1530/EJE-19-0762
Colao A, Bronstein MD, Freda P, Gu F, Shen CC, Gadelha M, Fleseriu M, van der Lely AJ, Farrall AJ, Hermosillo Reséndiz K, Ruffin M, Chen Y, Sheppard M, Pasireotide C2305 Study Group (2014) Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J Clin Endocrinol Metab 99:791–799. https://doi.org/10.1210/jc.2013-2480
Adigun OO, Nguyen M, Fox TJ, Anastasopoulou C. Acromegaly. StatPearls. Treasure Island (FL): Statpearls Publishing Copyright© 2021, StatPearls Publishing LLC; 2021. https://www.ncbi.nlm.nih.gov/books/NBK431086/. Accessed on Mar 1 2022.
Ambrosio MR, Gagliardi I, Chiloiro S, Ferreira AG, Bondanelli M, Giampietro A, Bianchi A, Marinis L, Fleseriu M, Zatelli MC (2020) Acromegaly in the elderly patients. Endocrine 68:16–31. https://doi.org/10.1007/s12020-020-02206-7
Andreasi V, Partelli S, Muffatti F, Manzoni MF, Capurso G, Falconi M (2021) Update on gastroenteropancreatic neuroendocrine tumors. Dig Liver Dis 53:171–182. https://doi.org/10.1016/j.dld.2020.08.031
Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM (2011) The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 40(1):1–18. https://doi.org/10.1016/j.ecl.2010.12.005
Kunz PL (2015) Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol 33:1855–1863. https://doi.org/10.1200/JCO.2014.60.2532
Reid TJ, Post KD, Bruce JN, Nabi Kanibir M, Reyes-Vidal CM, Freda PU (2010) Features at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosed. Clin Endocrinol (Oxf) 72:203–208. https://doi.org/10.1111/j.1365-2265.2009.03626.x
Mpilla GB, Philip PA, El-Rayes B, Azmi AS (2020) Pancreatic neuroendocrine tumors: therapeutic challenges and research limitations. World J Gastroenterol 26:4036–4054. https://doi.org/10.3748/wjg.v26.i28.4036
Ahmed A, Turner G, King B, Jones L, Culliford D, McCance D, Ardill J, Johnston BT, Poston G, Rees M, Buxton-Thomas M, Caplin M, Ramage JK (2009) Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 16:885–894. https://doi.org/10.1677/ERC-09-0042
Alhawyan FS (2021) Mortality in acromegalic patients: etiology, trends, and risk factors. Cureus 13:e14265. https://doi.org/10.7759/cureus.14265
Yang H, Tan H, Huang H, Li J (2021) Advances in research on the cardiovascular complications of acromegaly. Front Oncol 11:640999. https://doi.org/10.3389/fonc.2021.640999
Ni K, Yang JY, Baeg K, Leiter AC, Mhango G, Gallagher EJ et al (2021) Association between somatostatin analogues and diabetes mellitus in gastroenteropancreatic neuroendocrine tumor patients: a surveillance, epidemiology, and end results-medicare analysis of 5235 patients. Cancer Rep (Hoboken) 4:e1387. https://doi.org/10.1002/cnr2.1387
Cozzolino A, Feola T, Simonelli I, Puliani G, Pozza C, Giannetta E, Gianfrilli D, Pasqualetti P, Lenzi A, Isidori AM (2018) Somatostatin analogs and glucose metabolism in acromegaly: a meta-analysis of prospective interventional studies. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2017-02566
Moretti P, Dennis JL, Stella A, Alpini A, Cotichelli P, Ferolla P, Scarpelli G, Quartesan R, Piselli M (2013) Comorbilità di ansia e depressione nei pazienti con tumori carcinoidi [Comorbility between anxiety and depression in patients with carcinoid tumors]. Riv Psichiatr 48:301–306. https://doi.org/10.1708/1319.14626
Ministerio de Salud y Protección Social. Plan de beneficios en salud. Resolución 2292 de 2021 y sus anexos. Bogotá. Colombia.2021. https://www.minsalud.gov.co/Normatividad_Nuevo/Resoluci%C3%B3n%20No.%202292%20de%202021.pdf
Carmichael JD, Bonert VS, Nuño M, Ly D, Melmed S (2014) Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab 99:1825–1833. https://doi.org/10.1210/jc.2013-3757
Chanson P (2016) Medical treatment of acromegaly with dopamine agonists or somatostatin analogs. Neuroendocrinology 103:50–58. https://doi.org/10.1159/000377704
Ronchi CL, Rizzo E, Lania AG, Pivonello R, Grottoli S, Colao A, Ghigo E, Spada A, Arosio M, Beck-Peccoz P (2008) Preliminary data on biochemical remission of acromegaly after somatostatin analogs withdrawal. Eur J Endocrinol 158:19–25. https://doi.org/10.1530/EJE-07-0488
Ramírez C, Vargas G, González B, Grossman A, Rábago J, Sosa E, Espinosa-de-Los-Monteros AL, Mercado M (2012) Discontinuation of octreotide LAR after long term, successful treatment of patients with acromegaly: is it worth trying? Eur J Endocrinol 166:21–26. https://doi.org/10.1530/EJE-11-0738
Broder MS, Cai B, Chang E, Yan T, Benson AB 3rd (2018) First-line systemic treatment adherence, healthcare resource utilization, and costs in patients with gastrointestinal neuroendocrine tumors (GI NETs) in the USA. J Med Econ 21:821–826. https://doi.org/10.1080/13696998.2018.1474748
Funding
Open Access funding provided by Colombia Consortium. This study has been funded by Sanofi Colombia S.A and IPSEN Export Latin America, including editorial support and medical writing (Grant Number: E003183552).
Author information
Authors and Affiliations
Contributions
MEMD: conceptualization, methodology, formal analysis, investigation, data curation, and writing the original draft. AGM: formal analysis, investigation, data curation. JEMA: methodology, validation, formal analysis, resources, writing, review and editing, and supervision. LB: investigation, resources; LT: investigation, resources; CC: investigation, resources; IA: LB: investigation, resources.
Corresponding author
Ethics declarations
Conflict of interest
LB, LT, CC are employees of Sanofi-Aventis Colombia, and may hold shares and/or stock options in the company. IA is an employee of Ipsen. JEMA, AGM and MEMD are employees of Audifarma and Universidad Tecnologica de Pereira and do not have any other conflict of interest.
Ethics approval and consent to participate
The protocol was approved by the Bioethics Committee of the Universidad Tecnológica de Pereira (Technological University of Pereira) in the category of risk-free research (Code: 20 - 150321). The ethical principles established by the Declaration of Helsinki were respected.
Informed consent
The information was obtained from an administrative database. According to Colombian regulations (Resolution 8430 of 1993 of the Ministry of Health) for risk-free research in which patients are not interviewed, it is not required to sign an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machado-Alba, J.E., Machado-Duque, M.E., Gaviria-Mendoza, A. et al. Prescription patterns of somatostatin analogs in patients with acromegaly and neuroendocrine tumors. J Endocrinol Invest 46, 27–35 (2023). https://doi.org/10.1007/s40618-022-01875-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01875-7