Abstract
Background
P450 oxidoreductase (POR) deficiency (PORD) is characterized by congenital adrenal hyperplasia (CAH) and disorders of sex development (DSD) in both sexes. PORD can also associate with skeletal defects. However, the prevalence of these phenotypes is unknown.
Aim
To evaluate the prevalence of CAH, DSD, and infertility of patients with POR gene pathogenic variants by a systematic review of the literature.
Methods
The literature search was performed through PubMed, MEDLINE, Cochrane, Academic One Files, Google Scholar, and Scopus databases. All studies reporting information on CAH, DSD, testicular adrenal rest tumor (TARTs), and fertility in patients with POR gene pathogenic variants were included. Finally, the prevalence of abnormal phenotypes was calculated.
Results
Of the 246 articles initially retrieved, only 48 were included for a total of 119 (46 males and 73 females) patients with PORD. We also included the case of a male patient who consulted us for CAH and TARTs but without DSD. This patient, found to be a carrier of combined heterozygous POR mutation, reached fatherhood spontaneously. All the patients found had CAH. The presence of DSD was found in 65.2%, 82.1%, and 82.1% of patients with compound heterozygosity, homozygosity, or monoallelic heterozygous variants, respectively. The prevalence was significantly higher in females than in males. The prevalence of TARTs in patients with PORD is 2.7%. Only 5 women with PORD became pregnant after assisted reproductive techniques and delivered a healthy baby. Except for the recently reported proband, no other studies focused on male infertility in patients with POR gene variants.
Conclusion
This systematic review of the literature reports the prevalence of CAH, DSD, and TARTs in patients with PORD. The unknown prevalence of POR gene pathogenetic variants and the paucity of studies investigating fertility do not allow us to establish whether PORD is associated with infertility. Further studies on both women and men are needed to clarify this relationship.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital adrenal hyperplasia (CAH) describes a group of autosomal recessive disorders of cortisol biosynthesis with varying levels of severity [1]. Indeed, the clinical phenotype is typically classified as a classic form (the most severe one), and non-classic that is mild or late onset. Disorders of sex development (DSD) depend on the affected gene that cause CAH but also on sex proband. The presence of salt-wasting, postnatal virilization, sex steroid deficiency, hypertension, and other features, such as skeletal defects, are caused by the specific gene that is mutated [1]. Among the long-term complications of CAH, infertility is frequent in both female and male patients [2,3,4,5,6,7,8]. Male patients may experience the growth of testicular adrenal rest tumors (TARTs), which can cause intra-testicular compression of efferent seminiferous tubules in turn affecting spermatogenesis [9].
CAHs have a specific hormonal pattern based on the enzymatic dysfunction that causes it. The CYP21A2 genotype is the main, but not the only, determinant of the phenotype in patients with 21α-hydroxylase deficiency (21α-OHD) [10]. In some patients suspected of having a 21α-OHD, no pathogenic variants were found in one or both alleles even after complete sequencing of the CYP21A2 gene [11]. Other patients have pathogenetic variants of the CYP21A2 gene, but the genotype does not fully match the phenotype. These apparent discrepancies between genotype and phenotype suggest the presence of other genetic factors including modifier genes that can modulate the clinical expression of some aspects of 21α-OHD [10].
In humans, P450 oxidoreductase (POR) deficiency (PORD) causes an unusual and rare form of CAH, whose exact incidence is not known [12]. POR is an 82-kDa membrane-bound protein containing 680 residues encoded by a 32-kb gene containing 15 exons mapping on chromosome 7q11.2 [13]. It is necessary for the metabolic activity of P450 cytochrome enzymes including CYP17A1, CYP21A2, CYP19A1, and CYP51A1 [14]. Consequently, PORD can affect the function of these enzymes with different phenotype based on the residual enzymatic activity. As an example, studies on the CYP17A1, the steroidogenic enzyme that catalyzes both 17α-hydroxylase and 17,20 lyase activities [15], show that the levels of some P450 activities are determined, at least in part, by the stereochemistry of the interaction of POR with the cytochrome P450 [13]. In the case of CYP17A1, the 17,20 lyase reaction, but not the 17α-hydroxylase reaction, is very sensitive to this stereochemistry, as shown by three lines of evidence [13]. First, variants of basic residues in the redox-partner binding site of CYP17A1 selectively reduce the 17,20 lyase activity [16]. Second, cytochrome b5 acts as an allosteric factor to promote the interaction of P450c17 with POR, selectively increasing 17,20 lyase activity [17,18,19]. Third, phosphorylation of CYP17A1 selectively increases the 17,20 lyase activity of CYP17A1 [19,20,21].
PORD phenotype is characterized by DSD in both sexes and is often associated with skeletal defects [13, 22]. The latter, known as Antley–Bixler syndrome (ABS), is characterized by craniosynostosis, brachycephaly, radio-ulnar or radio-humeral synostosis, bowed femora, arachnodactyly, midface hypoplasia, proptosis, and choanal stenosis. ABS is transmitted with an autosomal recessive mechanism by POR pathogenic variants and with an autosomal dominant mechanism by gain-of-function variants of the fibroblast growth factor receptor 2 (FGFR2) gene [23]. No definitive data are available on TARTs and fertility in male patients with recessive PORD.
Therefore, this study aimed to systematically review the literature to gather all the available information on gender and genotype-related prevalence of CAH, DSD, TARTs, and fertility in patients with heterozygous or homozygous POR gene variants. In the resulting database, we added also the case of a male patient with CAH and TARTs but without DSD. He resulted in being a carrier of a combined heterozygous POR pathogenic variant and achieved fatherhood spontaneously.
Systematic review of the literature
Methods
Sources
Data for the systematic review were independently extracted by C.G. and R.C. A systematic search was performed through PubMed, MEDLINE, Cochrane, Academic One Files, Google Scholar, and Scopus databases, from the beginning of each database through May 22, 2021. The search strategy was based on the combination of the following Medical Subjects Headings (MeSH) terms and keywords, using “AND” between each MeSH search term: “P450 oxidoreductase” AND “congenital adrenal hyperplasia”, “P450 oxidoreductase” AND “DSD”, “P450 oxidoreductase” AND “homozygosity”, “P450 oxidoreductase” AND “heterozygosity”, “P450 oxidoreductase” AND “pregnancy”. Additional manual searches were made using the reference lists of relevant studies. Only articles available in English full-text have been included.
Study selection
All studies that reported the clinical phenotype of patients with POR gene variants were included. In particular, we focused on the presence of CAH, DSD (defined as virilization in female patients, under-masculinization in male patients, and abnormalities of the reproductive system in both sexes), TARTs, and fertility. Review articles and studies on experimental animals were excluded.
Description of the proband
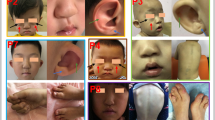
We added to our database the case of a patient not previously described in the literature who resulted in a carrier of compound heterozygous c.1891G > A, p. (Val631Ile) and c.516G > A variant. He had classical salt-wasting CAH, TARTs (Fig. 1), infertility, abnormal sperm parameters (oligoasthenoteratozoospermia, OAT), and extremely elevated ACTH serum levels. He achieved spontaneous paternity after adding dexamethasone 0.25 mg/day to his daily cortisol replacement therapy. This therapeutic arrangement led to the normalization of ACTH serum levels.
Results
Using the above-mentioned search terms, we found 246 articles. After the exclusion of 34 duplicated records, 212 articles were screened. Thirty-one articles were excluded because the English full-text was not available. Of the remaining articles, 119 were excluded after having read their title and abstract, since they did not satisfy the inclusion criteria. In particular, 10 studies were excluded because performed in vitro and/or on animals, and 24 were excluded because they were reviews. The remaining 62 full-texts were carefully read. Finally, 48 articles matched the inclusion criteria [14, 22, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. These studies included 119 patients (46 males and 73 females) with PORD (Fig. 2). They had homozygous, combined, or heterozygous POR gene variants in 58.0% (69/119), 32.8% (39/119), and 9.2% (11/119), respectively. These patients are worldwide distributed. The main features of the included studies are summarized in Table 1.
Congenital adrenal hyperplasia
The results showed that all the 119 patients with PORD had CAH. All of them showed increased serum 17α-hydroxyprogesterone levels at baseline or after the ACTH-stimulation test. Hence, no difference in the prevalence of CAH among homozygous, combined, or heterozygous POR gene pathogenetic variant was found (Table 1).
Disorder of sex development
DSD was described in 85 out of the 119 patients (71.4%) but there was a different genotype (homozygous or heterozygous) and gender-related distribution. DSD was found in 65.2% (45/69) of patients with compound heterozygous variants, 82.1% (32/39) of patients with homozygous variants, and 72.7% (8/11) of those with monoallelic heterozygous variants. Moreover, DSD occurred more frequently in females affecting 79.5% (58/73) of them and 58.7% (27/46) male patients (p < 0.05).
Testicular adrenal rest tumors
Out of 119 patients retrieved in the literature, ultrasound evaluation was performed only in 15 male probands without evidence of TARTs. Considering the case reported here, the prevalence of TARTs in PORD is, therefore, 6.6% (1/15), taking into account only patients who have undergone testicular ultrasound screening.
Fertility
Considering the higher prevalence of pediatric probands found in the literature, we found an important limitation in the assessment of fertility in patients with PORD. Of these, only 27 probands were over the age of 18 and, therefore, eligible for fertility research. No studies had focused on fertility in patients with PORD until 2017. To date, only five studies of female patients with PORD who have successfully delivered after ART-induced pregnancy have been published [57, 62, 63, 69].
Discussion
By reviewing data from 48 articles including 119 patients from around the world, the present study aims to show the gender- and genotype-related prevalence of CAH, DSD, TARTs, and infertility in patients with heterozygous or homozygous POR gene variants. Genotype–phenotype correlation is sometimes a complex and attempted association and is still a matter of research, considering the most recent studies that have focused on mutations of the CYP21A2 gene [70, 71]. This review allows a better understanding of the PORD phenotype. Our results show that CAH can be caused by both homozygous and heterozygous POR gene variants. Among 119 patients DSD was found in 85 (71.4%), respectively, in 65.2% (45/69) of patients with compound heterozygous variants, 82.1% (32/39) of patients with homozygous variants (82.1%), and 72.7% (8/11) of those with monoallelic heterozygous variants. Furthermore, DSD had a higher frequency in females affecting 79.5% (58/73) and 58.7% (27/46) male patients (p < 0.05), indicating that DSD affects both homozygous and heterozygous carrier patients, although with a higher prevalence in the former.
Specifically analyzing the individual variants, the most frequent are c.859G > C (p.A287P), typically with a higher prevalence of DSD when the heterozygous mutation is found in association with another monoallelic variant (DSD present in 81% of probands with compound heterozygous variants); c.1370G > A(p.R457H), that shows a higher prevalence of DSD when the homozygous variants are found (79%); c.1697G > A(p.G539R), with no difference between homozygous or heterozygous pathogenic variants (DSD present in 100% of probands) (Table 2). All the other detected variants, including the one found in our proband, were found in 1–2 patients (for each variant), so statistical analysis could not be applied (Table 3). According to a recent publication on POR polymorphisms, variants of this gene can influence CAH phenotypical expression acting as a genetic modifier of CYP21A2 defects [72]. Although the literature has so far focused on different allelic mutations on a single gene, we should now consider the coexistence of POR variants and polymorphisms in CAH patients carrying CYP21A2, CYP11B1, CYP17A1, HSD3B2, StAR, or CYP11A1 variants.
This is the first article reporting the presence of TARTs in a patient with CAH due to PORD. So far, TARTs have been described in patients with 21α-OHD and 11ß-hydroxylase deficiency [73, 74]. It is thought that poor hormonal control, leading to high blood levels of ACTH, is an important factor in the pathogenesis of TARTs by inducing hypertrophy and hyperplasia of these adrenal-like cells within the testis [75, 76]. Accordingly, the case herein reported showed that TARTs tend to grow when ACTH levels are elevated. However, TARTs are also found in properly treated patients, whereas some poorly controlled CAH patients never develop TARTs despite they are chronically exposed to elevated ACTH levels [77, 78]. The most plausible explanation for this observation is that in the embryological period aberrant adrenal cells do not nestle in the testes in all males, so the presence of these aberrant adrenal cells within the testis is a prerequisite for the development of TARTs [73]. This ectopic migration does not seem to be related to a specific genotype as so far described. However, the presence of TARTs in the patients described in this article allows us to speculate that also POR gene variants, can cause TART development. Articles on PORD patients do not report the presence of TARTs in any of the patients described.
The present study shows, for the first time, spontaneous fatherhood in a patient with CAH and TARTs due to variants of the POR gene, although paternity must to be genetically proven once the child is born. Female patients, on the other side, may have infertility due to increased androgen secretion or impaired sex steroid production [79], but few cases of successful births by ART-induced pregnancy have been reported [57, 62, 63, 69]. This observation suggests that women with PORD must undergo ART programs to achieve pregnancy.
In conclusion, the unknown incidence of POR gene variants and the poorness of fertility-investigating reports enlighten that it is still unclear whether PORD is associated with human infertility since most of the cases reported so far did not focus on patients’ fertility. Further studies exploring the relationship between POR genotype and fertility are needed. Evidence from the male proband herein reported suggests that spontaneous fatherhood can occur in male patients with PORD but proper management of CAH is needed to reach spontaneous fertility without the need to undergo ART.
References
Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, Arlt W, Auchus RJ, Falhammar H, Flück CE, Guasti L, Huebner A, Kortmann B et al (2022) Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocrine Rev 43(1):91–159
Jääskeläinen J, Kiekara O, Hippeläinen M, Voutilainen R (2000) Pituitary gonadal axis and child rate in males with classical 21-hydroxylase deficiency. J Endocrinol Invest 23(1):23–27
Stikkelbroeck NM, Otten BJ, Pasic A, Jager GJ, Sweep CG, Noordam K, Hermus AR (2001) High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86(12):5721–5728
Reisch N, Flade L, Scherr M, Rottenkolber M, Pedrosa Gil F, Bidlingmaier M, Wolff H, Schwarz HP, Quinkler M, Beuschlein F, Reincke M (2009) High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab 94(5):1665–1670
Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA et al (2010) United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE). Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab 95(11):5110–5121
Falhammar H, Nyström HF, Ekström U, Granberg S, Wedell A, Thorén M (2012) Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol 166(3):441–449
Bouvattier C, Esterle L, Renoult-Pierre P, de la Perrière AB, Illouz F, Kerlan V, Pascal-Vigneron V, Drui D, Christin-Maitre S et al (2015) Clinical outcome, hormonal status, gonadotropic axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French National Survey. J Clin Endocrinol Metab 100(6):2303–2313
King TF, Lee MC, Williamson EE, Conway GS (2016) Experience in optimizing fertility outcomes in men with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. Clin Endocrinol (Oxf) 84(6):830–836
Rohayem J, Bäumer LM, Zitzmann M, Fricke-Otto S, Mohnike K, Gohlke B, Reschke F, Jourdan C, Müller HL, Dunstheimer D et al (2021) Semen quality and testicular adrenal rest tumour development in 46, XY congenital adrenal hyperplasia: the importance of optimal hormonal replacement. Eur J Endocrinol 184(4):487–501
Baronio F, Ortolano R, Menabò S, Cassio A, Baldazzi L, Di Natale V, Tonti G, Vestrucci B, Balsamo A (2019) 46, XX DSD due to androgen excess in monogenic disorders of steroidogenesis: genetic, biochemical, and clinical features. Int J Mol Sci 20(18):4605
Reardon W, Smith A, Honour JW, Hindmarsh P, Das D, Rumsby G, Nelson I, Malcolm S, Adès L, Sillence D et al (2000) Evidence for digenic inheritance in some cases of Antley-Bixler syndrome? J Med Genet 37(1):26–32
Baranowski ES, Arlt W, Idkowiak J (2018) Monogenic disorders of adrenal steroidogenesis. Horm Res Paediatr 89(5):292–310
Miller WL, Huang N, Pandey AV, Flück CE, Agrawal V (2005) P450 oxidoreductase deficiency: a new disorder of steroidogenesis. Ann NY Acad Sci 1061:100–108
Parween S, Fernández-Cancio M, Benito-Sanz S, Camats N, Rojas Velazquez MN, López-Siguero JP, Udhane SS, Kagawa N, Flück CE, Audí L et al (2020) Molecular basis of CYP19A1 deficiency in a 46,XX patient with R550W mutation in POR: expanding the PORD phenotype. J Clin Endocrinol Metab 105(4):dgaa076
Miller WL, Auchus RJ, Geller DH (1997) The regulation of 17,20 lyase activity. Steroids 62(1):133–142
Geller DH, Auchus RJ, Mendonça BB, Miller WL (1997) The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genet 17(2):201–205
Auchus RJ, Lee TC, Miller WL (1998) Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273(6):3158–3165
Geller DH, Auchus RJ, Miller WL (1999) P450c17 mutations R347H and R358Q selectively disrupt 17,20-lyase activity by disrupting interactions with P450 oxidoreductase and cytochrome b5. Mol Endocrinol 13(1):167–175
Pandey AV, Miller WL (2005) Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem 280(14):13265–13271
Zhang LH, Rodriguez H, Ohno S, Miller WL (1995) Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92(23):10619–10623
Pandey AV, Mellon SH, Miller WL (2003) Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278(5):2837–2844
Huang N, Pandey AV, Agrawal V, Reardon W, Lapunzina PD, Mowat D, Jabs EW, Van Vliet G, Sack J, Flück CE et al (2005) Diversity and function of mutations in p450 oxidoreductase in patients with Antley-Bixler syndrome and disordered steroidogenesis. Am J Hum Genet 76(5):729–749
Miller WL (2018) Mechanisms in endocrinology: rare defects in adrenal steroidogenesis. Eur J Endocrinol 179(3):R125–R141
Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka Mankiewicz M, Hauffa BP, Malunowicz EM et al (2004) Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet 363(9427):2128–2135
Fluck CE, Tajima T, Pandey AV, Arlt W, Okuhara K, Verge CF, Jabs EW, Mendonca BB, Fujieda K, Miller WL (2004) Mutant P450 oxidoreductase causes disordered steroidogenesis with and without Antley-Bixler syndrome. Nat Genet 36(3):228–230
Adachi M, Tachibana K, Asakura Y, Yamamoto T, Hanaki K, Oka A (2004) Compound heterozygous mutations of cytochrome P450 oxidoreductase gene (POR) in two patients with Antley-Bixler syndrome. Am J Med Genet A 128A(4):333–339
Wudy SA, Hartmann MF, Draper N, Stewart PM, Arlt W (2004) A male twin infant with skull deformity and elevated neonatal 17-hydroxyprogesterone: a prismatic case of P450 oxidoreductase deficiency. Endocr Res 30(4):957–964
Shackleton C, Marcos J, Malunowicz EM, Szarras-Czapnik M, Jira P, Taylor NF, Murphy N, Crushell E, Gottschalk M, Hauffa B et al (2004) Biochemical diagnosis of Antley-Bixler syndrome by steroid analysis. Am J Med Genet A 128A(3):223–231
Fukami M, Horikawa R, Nagai T, Tanaka T, Naiki Y, Sato N, Okuyama T, Nakai H, Soneda S, Tachibana K et al (2005) Cytochrome P450 oxidoreductase gene mutations and Antley-Bixler syndrome with abnormal genitalia and/or impaired steroidogenesis: molecular and clinical studies in 10 patients. J Clin Endocrinol Metab 90(1):414–426
Fukami M, Hasegawa T, Horikawa R, Ohashi T, Nishimura G, Homma K, Ogata T (2006) Cytochrome P450 oxidoreductase deficiency in three patients initially regarded as having 21-hydroxylase deficiency and/or aromatase deficiency: diagnostic value of urine steroid hormone analysis. Pediatr Res 59(2):276–280
Homma K, Hasegawa T, Nagai T, Adachi M, Horikawa R, Fujiwara I, Tajima T, Takeda R, Fukami M, Ogata T (2006) Urine steroid hormone profile analysis in cytochrome P450 oxidoreductase deficiency: implication for the backdoor pathway to dihydrotestosterone. J Clin Endocrinol Metab 91(7):2643–2649
Williamson L, Arlt W, Shackleton C, Kelley RI, Braddock SR (2006) Linking Antley-Bixler syndrome and congenital adrenal hyperplasia: a novel case of P450 oxidoreductase deficiency. Am J Med Genet A 140A(17):1797–1803
Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL (2008) Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab 93(9):3584–3588
Nakamura N, Adachi M, Machida J, Okuzumi S (2008) Foot anomalies in Antley-Bixler syndrome: three case reports. J Pediatr Orthop B 17(5):241–245
Ko JM, Cheon CK, Kim GH, Yoo HW (2009) A case of Antley-Bixler syndrome caused by compound heterozygous mutations of the cytochrome P450 oxidoreductase gene. Eur J Pediatr 168(7):877–880
Sahakitrungruang T, Huang N, Tee MK, Agrawal V, Russell WE, Crock P, Murphy N, Migeon CJ, Miller WL (2009) Clinical, genetic, and enzymatic characterization of P450 oxidoreductase deficiency in four patients. J Clin Endocrinol Metab 94(12):4992–5000
Fukami M, Nishimura G, Homma K, Nagai T, Hanaki K, Uematsu A, Ishii T, Numakura C, Sawada H, Nakacho M et al (2009) Cytochrome P450 oxidoreductase deficiency: identification and characterization of biallelic mutations and genotype-phenotype correlations in 35 Japanese patients. J Clin Endocrinol Metab 94(5):1723–1731
Iijima S, Ohishi A, Ohzeki T (2009) Cytochrome P450 oxidoreductase deficiency with Antley–Bixler syndrome: steroidogenic capacities. J Pediatr Endocrinol Metab 22(5):469–475
Idkowiak J, Malunowicz EM, Dhir V, Reisch N, Szarras-Czapnik M, Holmes DM, Shackleton CH, Davies JD, Hughes IA, Krone N et al (2010) Concomitant mutations in the P450 oxidoreductase and androgen receptor genes presenting with 46, XY disordered sex development and androgenization at adrenarche. J Clin Endocrinol Metab 95(7):3418–3427
Tomalik-Scharte D, Maiter D, Kirchheiner J, Ivison HE, Fuhr U, Arlt W (2010) Impaired hepatic drug and steroid metabolism in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. Eur J Endocrinol 163(6):919–924
But WM, Lo IF, Shek CC, Tse WY, Lam ST (2010) Ambiguous genitalia, impaired steroidogenesis, and Antley-Bixler syndrome in a patient with P450 oxidoreductase deficiency. Hong Kong Med J 16(1):59–62
Idkowiak J, O’Riordan S, Reisch N, Malunowicz EM, Collins F, Kerstens MN, Köhler B, Graul-Neumann LM, Szarras-Czapnik M, Dattani M, Silink M et al (2011) Pubertal presentation in seven patients with congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab 96(3):E453–E462
Fluck CE, Mallet D, Hofer G, Samara-Boustani D, Leger J, Polak M, Morel Y, Pandey AV (2011) Deletion of P399_E401 in NADPH cytochrome P450 oxidoreductase results in partial mixed oxidase deficiency. Biochem Biophys Res Commun 412(4):572–577
Herkert JC, Blaauwwiekel EE, Hoek A, Veenstra-Knol HE, Kema IP, Arlt W, Kerstens MN (2011) A rare cause of congenital adrenal hyperplasia: Antley-Bixler syndrome due to POR deficiency. Neth J Med 69(6):281–283
Krone N, Reisch N, Idkowiak J, Dhir V, Ivison HE, Hughes BA, Rose IT, O’Neil DM, Vijzelaar R, Smith MJ et al (2012) Genotype-phenotype analysis in congenital adrenal hyperplasia due to P450 oxidoreductase deficiency. J Clin Endocrinol Metab 97(2):E257-267
Boia ES, Popoiu MC, Puiu M, Stanciulescu CM, David VL (2014) Antley-Bixler syndrome: surgical management of ambiguous genitalia—a case report. Med Princ Pract 23(4):384–386
Guaragna-Filho G, Castro CC, Carvalho RR, Coeli FB, Ferraz LF, Petroli RJ, Mello MP, Sewaybricker LE, Lemos-Marini SH, D’Souza-Li LF et al (2012) 46, XX DSD and Antley-Bixler syndrome due to novel mutations in the cytochrome P450 oxidoreductase gene. Arq Bras Endocrinol Metabol 56(8):578–585
Sánchez-Garvín D, Albaladejo S, Ezquieta B, Corripio R (2013) Disorder of sex development as a diagnostic clue in the first Spanish known newborn with P450 oxidoreductase deficiency. BMJ Case Rep 2013:bcr2013010251
Nakanishi K, Yamashita A, Miyamoto T, Takeguchi R, Furuya A, Matsuo K, Tanahashi Y, Kawamura M, Sengoku K (2016) P450 oxidoreductase deficiency with maternal virilization during pregnancy. Clin Exp Obstet Gynecol 43(6):902–904
Oldani E, Garel C, Bucourt M, Carbillon L (2015) Prenatal diagnosis of Antley-Bixler syndrome and POR deficiency. Am J Case Reports 16:882–885
Koika V, Armeni AK, Georgopoulos NA (2016) Delayed diagnosis of disorder of sex development (DSD) due to P450 oxidoreductase (POR) deficiency. Hormones (Athens) 15(2):277–282
Parween S, Roucher-Boulez F, Fluck CE, Lienhardt-Roussie A, Mallet D, Morel Y, Pandey AV (2016) P450 oxidoreductase deficiency: loss of activity caused by protein instability from a novel L374H mutation. J Clin Endocrinol Metab 101(12):4789–4798
Bonamichi BD, Santiago SL, Bertola DR, Kim CA, Alonso N, Mendonca BB, Bachega TA, Gomes LG (2016) Long-term follow-up of a female with congenital adrenal hyperplasia due to P450-oxidoreductase deficiency. Arch Endocrinol Metab 60(5):500–504
Tzetis M, Konstantinidou A, Sofocleous C, Kosma K, Mitrakos A, Tzannatos C, KitsiouTzeli S (2016) Compound heterozygosity of a paternal submicroscopic deletion and a maternal missense mutation in POR gene: Antley-Bixler syndrome phenotype in three sibling fetuses. Birth Defects Res A Clin Mol Teratol 106(7):536–541
Woo H, Ko JM, Shin CH, Yang SW (2016) Two cases of Antley-Bixler syndrome caused by mutations in different genes, FGFR2 and POR. J Genet Med 13(1):31–35
Bai Y, Li J, Wang X (2017) Cytochrome P450 oxidoreductase deficiency caused by R457H mutation in POR gene in Chinese: case report and literature review. J Ovarian Res 10(1):16
Song T, Wang B, Chen H, Zhu J, Sun H (2017) In vitro fertilization-frozen embryo transfer in a patient with cytochrome P450 oxidoreductase deficiency: a case report. Gynecol Endocrinol. https://doi.org/10.1080/09513590.2017.1393663
Khadilkar KS, Jagtap V, Lila A, Bandgar T, Shah NS (2017) Cytochrome P450 oxidoreductase deficiency: novel cause of ambiguity with primary amenorrhea. Indian J Endocrinol Metab 21(2):360–362
Oh J, Song JS, Park JE, Jang SY, Ki CS, Kim DK (2017) A case of Antley-Bixler syndrome with a novel likely pathogenic variant (c.529G>C) in the POR gene. Ann Lab Med 37(6):559–562
Fan L, Ren X, Song Y, Su C, Fu J, Gong C (2019) Novel phenotypes and genotypes in Antley-Bixler syndrome caused by cytochrome P450 oxidoreductase deficiency: based on the first cohort of Chinese children. Orphanet J Rare Dis 14(1):299
Lee Y, Choi JH, Oh A, Kim GH, Park SH, Moon JE, Ko CW, Cheon CK, Yoo HW (2020) Clinical, endocrinological, and molecular features of four Korean cases of cytochrome P450 oxidoreductase deficiency. Ann Pediatr Endocrinol Metab 25(2):97–103
Papadakis GE, Dumont A, Bouligand J, Chasseloup F, Raggi A, Catteau-Jonard S, Boute-Benejean O, Pitteloud N, Young J, Dewailly D (2020) Non-classic cytochrome P450 oxidoreductase deficiency strongly linked with menstrual cycle disorders and female infertility as primary manifestations. Hum Reprod 35(4):939–949
Zhang T, Li Z, Ren X, Huang B, Zhu G, Yang W, Jin L (2020) Clinical and genetic analysis of cytochrome P450 oxidoreductase (POR) deficiency in a female and the analysis of a novel POR intron mutation causing alternative mRNA splicing : Overall analysis of a female with POR deficiency. J Assist Reprod Genet 37(10):2503–2511
Aljabri A, Alnaim F, Alsaleh Y (2020) Combined homozygous 21 hydroxylase with heterozygous P450 oxidoreductase mutation in a Saudi boy presented with hypertension. BMJ Case Rep 13(9):e233942
Song F, Feng S, Shen X, Du M, Yin H, Liu R, Chen X (2020) Next-generation sequencing revealed disease-causing variants in two genes in a patient with combined features of spherocytosis and Antley-Bixler syndrome with genital anomalies and disordered steroidogenesis. Front Genet 21(11):976
Unal E, Demiral M, Yıldırım R, Taş FF, Ceylaner S, Özbek MN (2021) Cytochrome P450 oxidoreductase deficiency caused by a novel mutation in the POR gene in two siblings: case report and literature review. Hormones (Athens) 20(2):293–298
Wang W, Han R, Yang Z, Zheng S, Li H, Wan Z, Qi Y, Sun S, Ye L, Ning G (2021) Targeted gene panel sequencing for molecular diagnosis of congenital adrenal hyperplasia. J Steroid Biochem Mol Biol 14(211):105899
Rakover YT, Admoni O, Elias-Assad G, London S, Barhoum MN, Ludar H, Almagor T, Zehavi Y, Sultan C, Bertalan R et al (2021) The evolving role of whole-exome sequencing in the management of disorders of sex development. Endocr Connect EC-21-0019.R2
Pan P, Zheng L, Chen X, Huang J, Yang D, Li Y (2021) Successful live birth in a Chinese woman with P450 oxidoreductase deficiency through frozen-thawed embryo transfer: a case report with review of the literature. J Ovarian Res 14(1):22
Karaoğlan M, Nacarkahya G, Aytaç EH, Keskin M (2021) Challenges of CYP21A2 genotyping in children with 21-hydroxylase deficiency: determination of genotype-phenotype correlation using next generation sequencing in Southeastern Anatolia. J Endocrinol Invest 44(11):2395–2405
Mahmoud RAA, Amr NH, Toaima NN, Kamal TM, Elsedfy HH (2022) Genotypic spectrum of 21-hydroxylase deficiency in an endogamous population. J Endocrinol Invest 45(2):347–359
Pecori Giraldi F, Einaudi S, Sesta A, Verna F, Messina M, Manieri C, Menegatti E, Ghizzoni L (2021) POR polymorphisms are associated with 21 hydroxylase deficiency. J Endocrinol Invest 44(10):2219–2226
Dumic M, Duspara V, Grubic Z, Oguic SK, Skrabic V, Kusec V (2017) Testicular adrenal rest tumors in congenital adrenal hyperplasia-cross-sectional study of 51 Croatian male patients. Eur J Pediatr 176(10):1393–1404
El-Maouche D, Arlt W, Merke DP (2017) Congenital adrenal hyperplasia. Lancet 390(10108):2194–2210. https://doi.org/10.1016/S0140-6736(17)31431-9 (Epub 2017 May 30. Erratum in: Lancet. 2017 Nov 11;390(10108):2142)
Bonaccorsi AC, Adler I, Figueiredo JG (1987) Male infertility due to congenital adrenal hyperplasia: testicular biopsy findings, hormonal evaluation, and therapeutic results in three patients. Fertil Steril 47:664–670
Clark RV, Albertson BD, Munabi A et al (1990) Steroidogenic enzyme activities, morphology and receptor studies of a testicular adrenal rest in a patient with congenital adrenal hyperplasia. J Clin Endocrinol Metab 70:1408–1413
Cabrera MS, Vogiatzi MG, New MI (2001) Long-term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:3070–3078
Stikkelbroeck NMML, Otten BJ, Pasic A et al (2001) High prevalence of testicular adrenal rest tumours, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:5721–5728
Gomes LG, Bachega TASS, Mendonca BB (2019) Classic congenital adrenal hyperplasia and its impact on reproduction. Fertil Steril 111(1):7–12
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. This research did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research involving human participants and/or animals
This study was conducted at the Division of Endocrinology, Metabolic Diseases and Nutrition and Pediatric Endocrinology of the University-Teaching Hospital Policlinico “G. Rodolico”, University of Catania (Catania, Italy). The protocol was approved by the internal Institutional Review Board. The study has been conducted according to the principles expressed in the Declaration of Helsinki.
Informed consent
Informed consent was obtained from the patient after a full explanation of the purpose and nature of all procedures used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gusmano, C., Cannarella, R., Crafa, A. et al. Congenital adrenal hyperplasia, disorders of sex development, and infertility in patients with POR gene pathogenic variants: a systematic review of the literature. J Endocrinol Invest 46, 1–14 (2023). https://doi.org/10.1007/s40618-022-01849-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-022-01849-9