Abstract
Purpose
An increased fracture risk is commonly reported in Duchenne muscular dystrophy (DMD). Our aim was to investigate bone mineral density (BMD) and bone turnover, including sclerostin, and their association with markers of cardiac and respiratory performance in a cohort of DMD subjects.
Methods
In this single center, cross sectional observational study, lumbar spine (LS) BMD Z-scores, C-terminal telopeptide of procollagen type I (CTX) and osteocalcin (BGP), as bone resorption and formation markers, respectively, and sclerostin were assessed. Left ventricular ejection fraction (LVEF) and forced vital capacity (FVC) were evaluated. Clinical prevalent fractures were also recorded.
Results
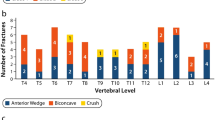
Thirty-one patients [median age = 14 (12–21.5) years] were studied. Ambulant subjects had higher LS BMD Z-scores compared with non-ambulant ones and subjects with prevalent clinical fractures [n = 9 (29%)] showed lower LS BMD Z-scores compared with subjects without fractures. LS BMD Z-scores were positively correlated with FVC (r = 0.50; p = 0.01), but not with glucocorticoid use, and FVC was positively associated with BGP (r = 0.55; p = 0.02). In non-ambulant subjects, LS BMD Z-scores were associated with BMI (r = 0.54; p = 0.02) and sclerostin was associated with age (r = 0.44; p = 0.05). Age, BMI, FVC and sclerostin were independently associated with LS BMD Z-score in a stepwise multiple regression analysis. Older age, lower BMI, FVC and sclerostin were associated with lower LS BMD Z-scores.
Conclusion
In a cohort of DMD patients, our data confirm low LS BMD Z-scores, mainly in non-ambulant subjects and irrespective of the glucocorticoid use, and suggest that FVC and sclerostin are independently associated with LS BMD Z-scores.

Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Dalkilic I, Kunkel LM (2003) Muscular dystrophies: genes to pathogenesis. Curr Opin Genet Dev 13(3):231–238. https://doi.org/10.1016/s0959-437x(03)00048-0
Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY (2016) Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003725.pub4
Birnkrant DJ, Bushby K, Bann CM et al (2018) Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17(4):347–361. https://doi.org/10.1016/S1474-4422(18)30025-5
Mayo AL, Craven BC, McAdam LC, Biggar WD (2012) Bone health in boys with Duchenne muscular dystrophy on long-term daily deflazacort therapy. Neuromuscul Disord 22(12):1040–1045. https://doi.org/10.1016/j.nmd.2012.06.354
Buckner JL, Bowden SA, Mahan JD (2015) Optimizing bone health in duchenne muscular dystrophy. Int J Endocrinol 2015:928385. https://doi.org/10.1155/2015/928385
Joseph S, Wang C, Bushby K et al (2019) Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment: results from the UK northstar database. JAMA Neurol 76(6):701–709. https://doi.org/10.1001/jamaneurol.2019.0242
Ma J, McMillan HJ, Karagüzel G et al (2017) The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int 28(2):597–608. https://doi.org/10.1007/s00198-016-3774-5
Rufo A, Del Fattore A, Capulli M et al (2011) Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res 26(8):1891–1903. https://doi.org/10.1002/jbmr.410
Boulanger Piette A, Hamoudi D, Marcadet L et al (2018) Targeting the muscle-bone unit: filling two needs with one deed in the treatment of Duchenne muscular dystrophy. Curr Osteoporos Rep 16(5):541–553. https://doi.org/10.1007/s11914-018-0468-2
Akhtar Ali S, Kang H, Olney R et al (2019) Evaluating RANKL and OPG levels in patients with Duchenne muscular dystrophy. Osteoporos Int 30(11):2283–2288. https://doi.org/10.1007/s00198-019-05077-5
Bianchi ML, Mazzanti A, Galbiati E et al (2003) Bone mineral density and bone metabolism in Duchenne muscular dystrophy. Osteoporos Int 14(9):761–767. https://doi.org/10.1007/s00198-003-1443-y
Bianchi ML, Morandi L, Andreucci E, Vai S, Frasunkiewicz J, Cottafava R (2011) Low bone density and bone metabolism alterations in Duchenne muscular dystrophy: response to calcium and vitamin D treatment. Osteoporos Int 22(2):529–539. https://doi.org/10.1007/s00198-010-1275-5
Söderpalm AC, Magnusson P, Ahlander AC et al (2007) Low bone mineral density and decreased bone turnover in Duchenne muscular dystrophy. Neuromuscul Disord 17(11–12):919–928. https://doi.org/10.1016/j.nmd.2007.05.008
Pouwels S, de Boer A, Leufkens HG et al (2014) Risk of fracture in patients with muscular dystrophies. Osteoporos Int 25(2):509–518. https://doi.org/10.1007/s00198-013-2442-2
Ferrari S, Bianchi ML, Eisman JA et al (2012) Osteoporosis in young adults: pathophysiology, diagnosis, and management. Osteoporos Int 23(12):2735–2748. https://doi.org/10.1007/s00198-012-2030-x
Larson CM, Henderson RC (2000) Bone mineral density and fractures in boys with Duchenne muscular dystrophy. J Pediatr Orthop 20(1):71–74
Bell JM, Shields MD, Watters J et al (2017) Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010899.pub2
Wong BL, Rybalsky I, Shellenbarger KC et al (2017) Long-term outcome of interdisciplinary management of patients with Duchenne muscular dystrophy receiving daily glucocorticoid treatment. J Pediatr 182:296-303.e1. https://doi.org/10.1016/j.jpeds.2016.11.078
Guañabens N, Gifre L, Peris P (2014) The role of Wnt signaling and sclerostin in the pathogenesis of glucocorticoid-induced osteoporosis. Curr Osteoporos Rep 12(1):90–97. https://doi.org/10.1007/s11914-014-0197-0
Moester MJ, Papapoulos SE, Löwik CW, van Bezooijen RL (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87(2):99–107. https://doi.org/10.1007/s00223-010-9372-1
Appelman-Dijkstra NM, Papapoulos SE (2016) Sclerostin inhibition in the management of osteoporosis. Calcif Tissue Int 98(4):370–380. https://doi.org/10.1007/s00223-016-0126-6
Winkler DG, Sutherland MK, Geoghegan JC et al (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276. https://doi.org/10.1093/emboj/cdg599
Li X, Ominsky MS, Niu QT et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869. https://doi.org/10.1359/jbmr.080216
Gaudio A, Pennisi P, Bratengeier C et al (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95(5):2248–2253. https://doi.org/10.1210/jc.2010-0067
McDonald CM, Henricson EK, Abresch RT et al (2018) Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 391(10119):451–461. https://doi.org/10.1016/S0140-6736(17)32160-8
Catalano A, Martino G, Morabito N, Scarcella C, Gaudio A, Basile G, Lasco A (2017) Pain in osteoporosis: from pathophysiology to therapeutic approach. Drugs Aging 34(10):755–765. https://doi.org/10.1007/s40266-017-0492-4 (PMID: 28980156)
Bianchi ML, Leonard MB, Bechtold S et al (2014) Bone health in children and adolescents with chronic diseases that may affect the skeleton: the 2013 ISCD pediatric official positions. J Clin Densitom 17(2):281–294. https://doi.org/10.1016/j.jocd.2014.01.005
Catalano A, Vita GL, Russo M et al (2016) Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporos Int 27(12):3655–3659. https://doi.org/10.1007/s00198-016-3761-x
Zemel BS, Leonard MB, Kelly A et al (2010) Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95(3):1265–1273. https://doi.org/10.1210/jc.2009-2057
Nasomyont N, Keefe C, Tian C et al (2020) Safety and efficacy of teriparatide treatment for severe osteoporosis in patients with Duchenne muscular dystrophy. Osteoporos Int 31(12):2449–2459. https://doi.org/10.1007/s00198-020-05549-z
Zheng WB, Dai Y, Hu J et al (2020) Effects of bisphosphonates on osteoporosis induced by Duchenne muscular dystrophy: a prospective study. Endocr Pract 26(12):1477–1485. https://doi.org/10.4158/EP-2020-0073
Ionescu AA, Schoon E (2003) Osteoporosis in chronic obstructive pulmonary disease. Eur Respir J Suppl 46:64s–75s. https://doi.org/10.1183/09031936.03.00004609
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18(10):1319–1328. https://doi.org/10.1007/s00198-007-0394-0
Watanabe R, Shiraki M, Saito M, Okazaki R, Inoue D (2018) Restrictive pulmonary dysfunction is associated with vertebral fractures and bone loss in elderly postmenopausal women. Osteoporos Int 29(3):625–633. https://doi.org/10.1007/s00198-017-4337-0
Catalano A, Morabito N, Basile G et al (2013) Fracture risk assessment in postmenopausal women referred to an Italian center for osteoporosis: a single day experience in Messina. Clin Cases Miner Bone Metab 10(3):191–194
Lasco A, Catalano A, Pilato A, Basile G, Mallamace A, Atteritano M (2014) Subclinical hypercortisol-assessment of bone fragility: experience of single osteoporosis center in Sicily. Eur Rev Med Pharmacol Sci 18(3):352–358
Ghazi M, Kolta S, Briot K, Fechtenbaum J, Paternotte S, Roux C (2012) Prevalence of vertebral fractures in patients with rheumatoid arthritis: revisiting the role of glucocorticoids. Osteoporos Int 23(2):581–587. https://doi.org/10.1007/s00198-011-1584-3
Zhukouskaya VV, Eller-Vainicher C, Gaudio A et al (2015) In postmenopausal female subjects with type 2 diabetes mellitus, vertebral fractures are independently associated with cortisol secretion and sensitivity. J Clin Endocrinol Metab 100(4):1417–1425. https://doi.org/10.1210/jc.2014-4177
Glass DA 2nd, Bialek P, Ahn JD et al (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8(5):751–764. https://doi.org/10.1016/j.devcel.2005.02.017
Catalano A, Morabito N, Basile G, Brancatelli S, Cucinotta D, Lasco A (2013) Zoledronic acid acutely increases sclerostin serum levels in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 98(5):1911–1915. https://doi.org/10.1210/jc.2012-4039
Ueland T, Stilgren L, Bollerslev J (2019) Bone matrix levels of Dickkopf and sclerostin are positively correlated with bone mass and strength in postmenopausal osteoporosis. Int J Mol Sci 20(12):2896. https://doi.org/10.3390/ijms20122896
Gao X, Tang Y, Amra S et al (2019) Systemic investigation of bone and muscle abnormalities in dystrophin/utrophin double knockout mice during postnatal development and the mechanisms. Hum Mol Genet 28(10):1738–1751. https://doi.org/10.1093/hmg/ddz012
Cairoli E, Zhukouskaya VV, Eller-Vainicher C, Chiodini I (2015) Perspectives on osteoporosis therapies. J Endocrinol Invest 38(3):303–311. https://doi.org/10.1007/s40618-014-0236-9
Rossini M, Gatti D, Adami S (2013) Involvement of WNT/β-catenin signaling in the treatment of osteoporosis. Calcif Tissue Int 93(2):121–132. https://doi.org/10.1007/s00223-013-9749-z
Tamanini S, Idolazzi L, Gatti D, Viapiana O, Fassio A, Rossini M (2013) Insight into the WNT system and its drug related response. Reumatismo 65(5):219–230. https://doi.org/10.4081/reumatismo.2013.219
Catalano A, Loddo S, Bellone F, Pecora C, Lasco A, Morabito N (2018) Pulsed electromagnetic fields modulate bone metabolism via RANKL/OPG and Wnt/β-catenin pathways in women with postmenopausal osteoporosis: a pilot study. Bone 116:42–46. https://doi.org/10.1016/j.bone.2018.07.010
Morabito N, Catalano A, Gaudio A et al (2016) Effects of strontium ranelate on bone mass and bone turnover in women with thalassemia major-related osteoporosis. J Bone Miner Metab 34(5):540–546. https://doi.org/10.1007/s00774-015-0689-8
Davidson ZE, Walker KZ, Truby H (2012) Clinical review: do glucocorticosteroids alter vitamin D status? A systematic review with meta-analyses of observational studies. J Clin Endocrinol Metab 97(3):738–744. https://doi.org/10.1210/jc.2011-2757
Bian Q, McAdam L, Grynpas M, Mitchell J, Harrington J (2019) Increased rates of vitamin D insufficiency in boys with Duchenne muscular dystrophy despite higher vitamin D3 supplementation. Glob Pediatr Health. https://doi.org/10.1177/2333794X19835661
Mazziotti G, Formenti AM, Adler RA et al (2016) Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 54(3):603–611. https://doi.org/10.1007/s12020-016-1146-8
Meriggioli MN, Roubenoff R (2015) Prospect for pharmacological therapies to treat skeletal muscle dysfunction. Calcif Tissue Int 96(3):234–242. https://doi.org/10.1007/s00223-014-9926-8
van der Meijden K, Bravenboer N, Dirks NF et al (2016) Effects of 1,25(OH)2 D3 and 25(OH)D3 on C2C12 myoblast proliferation, differentiation, and myotube hypertrophy. J Cell Physiol 231(11):2517–2528. https://doi.org/10.1002/jcp.25388
Lasco A, Catalano A, Benvenga S (2012) Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med 172(4):366–367. https://doi.org/10.1001/archinternmed.2011.715
Oteri G, Cicciù M, Peditto M et al (2016) Does vitamin D3 have an impact on clinical and biochemical parameters related to third molar surgery. J Craniofac Surg 27(2):469–476. https://doi.org/10.1097/SCS.0000000000002389
Gembillo G, Cernaro V, Siligato R, Curreri F, Catalano A, Santoro D (2020) Protective role of Vitamin D in renal tubulopathies. Metabolites 10(3):115. https://doi.org/10.3390/metabo10030115
Catalano A, Morabito N, Atteritano M, Basile G, Cucinotta D, Lasco A (2012) Vitamin D reduces musculoskeletal pain after infusion of zoledronic acid for postmenopausal osteoporosis. Calcif Tissue Int 90(4):279–285. https://doi.org/10.1007/s00223-012-9577-6
Messina S, Vita GL, Sframeli M et al (2016) Health-related quality of life and functional changes in DMD: a 12-month longitudinal cohort study. Neuromuscul Disord 26(3):189–196. https://doi.org/10.1016/j.nmd.2016.01.003
Messina S, Vita GL (2018) Clinical management of Duchenne muscular dystrophy: the state of the art. Neurol Sci 39(11):1837–1845. https://doi.org/10.1007/s10072-018-3555-3
Catalano A, Martino G, Bellone F et al (2018) Anxiety levels predict fracture risk in postmenopausal women assessed for osteoporosis. Menopause 25(10):1110–1115. https://doi.org/10.1097/GME.0000000000001123
Mazzone ES, Pane M, Sormani MP et al (2013) 24 month longitudinal data in ambulant boys with Duchenne muscular dystrophy. PLoS ONE 8(1):e52512. https://doi.org/10.1371/journal.pone.0052512
Vita G, Vita GL, Musumeci O, Rodolico C, Messina S (2019) Genetic neuromuscular disorders: living the era of a therapeutic revolution. Part 2: diseases of motor neuron and skeletal muscle. Neurol Sci 40(4):671–681. https://doi.org/10.1007/s10072-019-03764-z
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733. https://doi.org/10.1007/s00198-006-0172-4
Acknowledgements
This work was partially supported by a SIOMMMS (Società Italiana dell’Osteoporosi, del Metabolismo Minerale e delle Malattie dello Scheletro) research grant (to A.C.).
Author information
Authors and Affiliations
Contributions
Conceptualization: AC, GLV, NM; methodology: AC, GLV, SM; investigation: AC, GLV, FB, MF, MGD, MLR, AG, SM; formal analysis: AC, GLV; writing—original draft preparation: AC, GLV, SM, writing—review and editing: AC, GLV, GV, SM, NM; supervision: GV, SM, NM. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Antonino Catalano, Gian Luca Vita, Federica Bellone, Maria Sframeli, Maria Grazia Distefano, Matteo La Rosa, Agostino Gaudio, Giuseppe Vita, Nunziata Morabito and Sonia Messina have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of Scientific Ethic Committee of the AOU Policlinico “G. Martino”, Messina, Italy (Prot. 20-12) and with the 1964 Helsinki declaration and its later amendments.
Informed consent
An informed consent was obtained from all participants included in the study or from their parents as appropriate.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nunziata Morabito and Sonia Messina are both senior authors.
Rights and permissions
About this article
Cite this article
Catalano, A., Vita, G.L., Bellone, F. et al. Bone health in Duchenne muscular dystrophy: clinical and biochemical correlates. J Endocrinol Invest 45, 517–525 (2022). https://doi.org/10.1007/s40618-021-01676-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01676-4