Abstract
Purpose
Adrenocortical carcinoma (ACC) is a rare disease with few therapeutic options. There is an urgency of new effective therapeutic options for these patients. The role of immune checkpoint inhibitors (ICI) in advanced ACC patients is still unclear.
Methods
We conducted a MEDLINE search using the following string: adrenocortical carcinoma and immunotherapy or checkpoint inhibitors.
Results
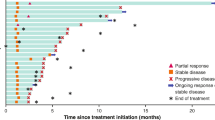
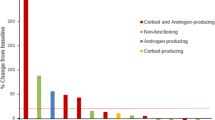
We found four case series comprising 10 patients, and four prospective studies totaling 115 patients. The response rate (RR) in the group of 10 patients was 1 complete response, 3 partial response (PR), 4 stable disease (SD), and 2 progressive disease (PD). The median progression-free survival (mPFS) ranged from 2 to 31 months and the median overall survival (mOS) ranged from 4.3 to 31 months. The results in the 115 patients from prospective trials was variable, the PR ranged from 6 to 23%, the SD ranged from 18 to 50% and overall disease control rate ranged from 30 to 64%. The mPFS reported varied from 1.8 to 2.6 months while the mOS varied from 10.6 to 24.9 months. There were five patients with sustained response for more than 24 months. The most common treatment-related adverse event (TRAE) was the increase in liver enzymes. No treatment-related deaths were reported. Better results in terms of RR and survival were observed in studies that used pembrolizumab. No predictive biomarker of response was found up to now.
Conclusion
ICI, mainly pembrolizumab, is a potential therapeutic option, which is safe and associated with prolonged OS benefit, in selected patients with advanced ACC.

Similar content being viewed by others
References
Vezzosi D, Do Cao C, Hescot S, Bertherat J, Haissaguerre M, Bongard V, Drui D, De La Fouchardiere C, Illouz F, Borson-Chazot F, Djobo B, Berdelou A, Tabarin A, Schlumberger M, Briet C, Caron P, Leboulleux S, Libe R, Baudin E, For Comete-Cancer N (2018) Time until partial response in metastatic adrenocortical carcinoma long-term survivors. Hormon Cancer 9(1):62–69. https://doi.org/10.1007/s12672-017-0313-6
Puglisi S, Perotti P, Pia A, Reimondo G, Terzolo M (2018) Adrenocortical carcinoma with hypercortisolism. Endocrinol Metab Clin N Am 47(2):395–407. https://doi.org/10.1016/j.ecl.2018.02.003
Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, Welin S, Schade-Brittinger C, Lacroix A, Jarzab B, Sorbye H, Torpy DJ, Stepan V, Schteingart DE, Arlt W, Kroiss M, Leboulleux S, Sperone P, Sundin A, Hermsen I, Hahner S, Willenberg HS, Tabarin A, Quinkler M, de la Fouchardiere C, Schlumberger M, Mantero F, Weismann D, Beuschlein F, Gelderblom H, Wilmink H, Sender M, Edgerly M, Kenn W, Fojo T, Muller HH, Skogseid B, Group F-AS (2012) Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med 366(23):2189–2197. https://doi.org/10.1056/NEJMoa1200966
Sperone P, Ferrero A, Daffara F, Priola A, Zaggia B, Volante M, Santini D, Vincenzi B, Badalamenti G, Intrivici C, Del Buono S, De Francia S, Kalomirakis E, Ratti R, Angeli A, Dogliotti L, Papotti M, Terzolo M, Berruti A (2010) Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer 17(2):445–453. https://doi.org/10.1677/ERC-09-0281
Henning JEK, Deutschbein T, Altieri B, Steinhauer S, Kircher S, Sbiera S, Wild V, Schlotelburg W, Kroiss M, Perotti P, Rosenwald A, Berruti A, Fassnacht M, Ronchi CL (2017) Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab 102(11):4323–4332. https://doi.org/10.1210/jc.2017-01624
Megerle F, Kroiss M, Hahner S, Fassnacht M (2019) Advanced adrenocortical carcinoma—what to do when first-line therapy fails? Exp Clin Endocrinol Diabetes 127(2–03):109–116. https://doi.org/10.1055/a-0715-1946
Grisanti S, Filice A, Basile V, Cosentini D, Rapa I, Albano D, Morandi A, Lagana M, Dalla Volta A, Bertagna F, Tiberio GMA, Volante M, Terzolo M, Versari A, Berruti A (2020) Treatment with 90Y/177Lu-DOTATOC in patients with metastatic adrenocortical carcinoma expressing somatostatin receptors. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz091
Kroiss M, Megerle F, Kurlbaum M, Zimmermann S, Wendler J, Jimenez C, Lapa C, Quinkler M, Scherf-Clavel O, Habra MA, Fassnacht M (2020) Objective response and prolonged disease control of advanced adrenocortical carcinoma with cabozantinib. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz318
Petrova V, Arkhypov I, Weber R, Groth C, Altevogt P, Utikal J, Umansky V (2020) Modern aspects of immunotherapy with checkpoint inhibitors in melanoma. Int J Mol Sci. https://doi.org/10.3390/ijms21072367
Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, Haydon A, Lichinitser M, Khattak A, Carlino MS, Sandhu S, Larkin J, Puig S, Ascierto PA, Rutkowski P, Schadendorf D, Koornstra R, Hernandez-Aya L, Maio M, van den Eertwegh AJM, Grob JJ, Gutzmer R, Jamal R, Lorigan P, Ibrahim N, Marreaud S, van Akkooi ACJ, Suciu S, Robert C (2018) Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378(19):1789–1801. https://doi.org/10.1056/NEJMoa1802357
Hanna NH, Schneider BJ, Temin S, Baker S Jr, Brahmer J, Ellis PM, Gaspar LE, Haddad RY, Hesketh PJ, Jain D, Jaiyesimi I, Johnson DH, Leighl NB, Phillips T, Riely GJ, Robinson AG, Rosell R, Schiller JH, Singh N, Spigel DR, Stabler JO, Tashbar J, Masters G (2020) Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol. https://doi.org/10.1200/JCO.19.03022
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro CJ, Faivre-Finn C, Reck M, Vansteenkiste J, Spigel DR, Wadsworth C, Melillo G, Taboada M, Dennis PA, Ozguroglu M, Investigators P (2018) Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 379(24):2342–2350. https://doi.org/10.1056/NEJMoa1809697
Le Tourneau C, Hoimes C, Zarwan C, Wong DJ, Bauer S, Claus R, Wermke M, Hariharan S, von Heydebreck A, Kasturi V, Chand V, Gulley JL (2018) Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer 6(1):111. https://doi.org/10.1186/s40425-018-0424-9
Carneiro BA, Konda B, Costa RB, Costa RLB, Sagar V, Gursel DB, Kirschner LS, Chae YK, Abdulkadir SA, Rademaker A, Mahalingam D, Shah MH, Giles FJ (2019) Nivolumab in metastatic adrenocortical carcinoma: results of a phase 2 trial. J Clin Endocrinol Metab 104(12):6193–6200. https://doi.org/10.1210/jc.2019-00600
Habra MA, Stephen B, Campbell M, Hess K, Tapia C, Xu M, Rodon Ahnert J, Jimenez C, Lee JE, Perrier ND, Boraddus RR, Pant S, Subbiah V, Hong DS, Zarifa A, Fu S, Karp DD, Meric-Bernstam F, Naing A (2019) Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J Immunother Cancer 7(1):253. https://doi.org/10.1186/s40425-019-0722-x
Raj N, Zheng Y, Kelly V, Katz SS, Chou J, Do RKG, Capanu M, Zamarin D, Saltz LB, Ariyan CE, Untch BR, O'Reilly EM, Gopalan A, Berger MF, Olino K, Segal NH, Reidy-Lagunes DL (2020) PD-1 Blockade in advanced adrenocortical carcinoma. J Clin Oncol 38(1):71–80. https://doi.org/10.1200/JCO.19.01586
Mota JM, Sousa LG, Braghiroli MI, Siqueira LT, Neto JEB, Chapchap P, Hoff AAO, Hoff PM (2018) Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden: two case reports. Medicine 97(52):e13517. https://doi.org/10.1097/MD.0000000000013517
Head L, Kiseljak-Vassiliades K, Clark TJ, Somerset H, King J, Raeburn C, Albuja-Cruz M, Weyant M, Cleveland J, Wierman ME, Leong S (2019) Response to immunotherapy in combination with mitotane in patients with metastatic adrenocortical cancer. J Endocr Soc 3(12):2295–2304. https://doi.org/10.1210/js.2019-00305
Casey RT, Giger O, Seetho I, Marker A, Pitfield D, Boyle LH, Gurnell M, Shaw A, Tischkowitz M, Maher ER, Chatterjee VK, Janowitz T, Mells G, Corrie P, Challis BG (2018) Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin Oncol 45(3):151–155. https://doi.org/10.1053/j.seminoncol.2018.06.001
Caccese M, Ceccato F, Fassan M, Fassina A, Padovan M, Mammi I, Iacobone M, Scaroni C, Zagonel V, Lombardi G (2019) Letter to Editor: Reply to R.T. Casey (Semin Oncol. 2018 Jun;45(3):151-155). Semin Oncol 46(1):104–105. https://doi.org/10.1053/j.seminoncol.2018.12.005
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15(23):7412–7420. https://doi.org/10.1158/1078-0432.CCR-09-1624
Naing A, Meric-Bernstam F, Stephen B, Karp DD, Hajjar J, Rodon Ahnert J, Piha-Paul SA, Colen RR, Jimenez C, Raghav KP, Ferrarotto R, Tu SM, Campbell M, Wang L, Sabir SH, Tapia C, Bernatchez C, Frumovitz M, Tannir N, Ravi V, Khan S, Painter JM, Abonofal A, Gong J, Alshawa A, McQuinn LM, Xu M, Ahmed S, Subbiah V, Hong DS, Pant S, Yap TA, Tsimberidou AM, Dumbrava EEI, Janku F, Fu S, Simon RM, Hess KR, Varadhachary GR, Amir Habra M (2020) Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000347
Cosentini D, Grisanti S, Dalla Volta A, Lagana M, Fiorentini C, Perotti P, Sigala S, Berruti A (2018) Immunotherapy failure in adrenocortical cancer: where next? Endocr Connect 7(12):E5–E8. https://doi.org/10.1530/EC-18-0398
Fiorentini C, Grisanti S, Cosentini D, Abate A, Rossini E, Berruti A, Sigala S (2019) Molecular drivers of potential immunotherapy failure in adrenocortical carcinoma. J Oncol 2019:6072863. https://doi.org/10.1155/2019/6072863
Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, Kroiss M, Quinn DI, Hesseltine E, Ronchi CL, Terzolo M, Choueiri TK, Poondru S, Fleege T, Rorig R, Chen J, Stephens AW, Worden F, Hammer GD (2015) Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol 16(4):426–435. https://doi.org/10.1016/S1470-2045(15)70081-1
Forde PM, Chaft JE, Pardoll DM (2018) Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 379(9):e14. https://doi.org/10.1056/NEJMc1808251
Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD, Illei PB, Gabrielson E, Askin FB, Niknafs N, Smith KN, Velez MJ, Sauter JL, Isbell JM, Jones DR, Battafarano RJ, Yang SC, Danilova L, Wolchok JD, Topalian SL, Velculescu VE, Pardoll DM, Brahmer JR, Hellmann MD, Chaft JE, Cimino-Mathews A, Taube JM (2018) Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 29(8):1853–1860. https://doi.org/10.1093/annonc/mdy218
Sepesi B, Cascone T (2020) Commentary: neoadjuvant checkpoint inhibitors in resectable non-small cell lung cancer-ready for prime time? J Thorac Cardiovasc Surg 159(4):1624–1625. https://doi.org/10.1016/j.jtcvs.2019.09.042
Goldfarb L, Duchemann B, Chouahnia K, Zelek L, Soussan M (2019) Monitoring anti-PD-1-based immunotherapy in non-small cell lung cancer with FDG PET: introduction of iPERCIST. EJNMMI Res 9(1):8. https://doi.org/10.1186/s13550-019-0473-1
Trebeschi S, Drago SG, Birkbak NJ, Kurilova I, Calin AM, Delli Pizzi A, Lalezari F, Lambregts DMJ, Rohaan MW, Parmar C, Rozeman EA, Hartemink KJ, Swanton C, Haanen J, Blank CU, Smit EF, Beets-Tan RGH, Aerts H (2019) Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann Oncol 30(6):998–1004. https://doi.org/10.1093/annonc/mdz108
Polverari G, Ceci F, Bertaglia V, Reale ML, Rampado O, Gallio E, Passera R, Liberini V, Scapoli P, Arena V, Racca M, Veltri A, Novello S, Deandreis D (2020) (18)F-FDG pet parameters and radiomics features analysis in advanced Nsclc treated with immunotherapy as predictors of therapy response and survival. Cancers. https://doi.org/10.3390/cancers12051163
Grecea M, Marabelle A, Ammari S, Massard C, Champiat S (2020) Managing hyperprogressive disease in the era of programmed cell death protein 1/programmed death-ligand 1 blockade: a case discussion and review of the literature. Oncologist 25(5):369–374. https://doi.org/10.1634/theoncologist.2019-0671
Libert C, Dejager L (2014) How steroids steer T cells. Cell Rep 7(4):938–939. https://doi.org/10.1016/j.celrep.2014.04.041
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martinez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, Sabari JK, Caramella C, Plodkowski AJ, Halpenny D, Chaft JE, Planchard D, Riely GJ, Besse B, Hellmann MD (2018) Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 36(28):2872–2878. https://doi.org/10.1200/JCO.2018.79.0006
Parra ER, Villalobos P, Behrens C, Jiang M, Pataer A, Swisher SG, William WN Jr, Zhang J, Lee J, Cascone T, Heymach JV, Forget MA, Haymaker C, Bernatchez C, Kalhor N, Weissferdt A, Moran C, Zhang J, Vaporciyan A, Gibbons DL, Sepesi B, Wistuba II (2018) Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J Immunother Cancer 6(1):48. https://doi.org/10.1186/s40425-018-0368-0
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, Investigators K (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. https://doi.org/10.1056/NEJMoa1801005
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM, Investigators K (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051. https://doi.org/10.1056/NEJMoa1810865
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O'Shaughnessy J, Investigators K (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/NEJMoa1910549
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Psyrri A, Baste N, Neupane P, Bratland A, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, Gonzalez Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D, Investigators K (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV, Group IMS (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229. https://doi.org/10.1056/NEJMoa1809064
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, Moiseyenko V, Antonia SJ, Le Moulec S, Robinet G, Natale R, Schneider J, Shepherd FA, Geater SL, Garon EB, Kim ES, Goldberg SB, Nakagawa K, Raja R, Higgs BW, Boothman AM, Zhao L, Scheuring U, Stockman PK, Chand VK, Peters S, Investigators M (2020) Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2020.0237
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520. https://doi.org/10.1056/NEJMoa1500596
Tierney JF, Vogle A, Poirier J, Min IM, Finnerty B, Zarnegar R, Pappas SG, Scognamiglio T, Ghai R, Gattuso P, Fahey TJ 3rd, Keutgen XM (2019) Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: an exploratory study. Surgery 165(1):196–201. https://doi.org/10.1016/j.surg.2018.04.086
Lang J, Capasso A, Jordan KR, French JD, Kar A, Bagby SM, Barbee J, Yacob BW, Head LS, Tompkins KD, Freed BM, Somerset H, Clark TJ, Pitts TM, Messersmith WA, Eckhardt SG, Wierman ME, Leong S, Kiseljak-Vassiliades K (2020) Development of an adrenocortical cancer humanized mouse model to characterize anti-PD1 effects on tumor microenvironment. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgz014
Liu J, Blake SJ, Yong MC, Harjunpaa H, Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ, Teng MW (2016) Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 6(12):1382–1399. https://doi.org/10.1158/2159-8290.CD-16-0577
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. https://doi.org/10.1038/nature13954
Zhang J, Ji Z, Caushi JX, El Asmar M, Anagnostou V, Cottrell TR, Chan HY, Suri P, Guo H, Merghoub T, Chaft JE, Reuss JE, Tam AJ, Blosser RL, Abu-Akeel M, Sidhom JW, Zhao N, Ha JS, Jones DR, Marrone KA, Naidoo J, Gabrielson E, Taube JM, Velculescu VE, Brahmer JR, Housseau F, Hellmann MD, Forde PM, Pardoll DM, Ji H, Smith KN (2020) Compartmental analysis of T-cell clonal dynamics as a function of pathologic response to neoadjuvant PD-1 blockade in resectable non-small cell lung cancer. Clin Cancer Res 26(6):1327–1337. https://doi.org/10.1158/1078-0432.CCR-19-2931
Fojo AT (2019) Immunotherapy for adrenocortical cancer. Semin Oncol 46(1):1–2. https://doi.org/10.1053/j.seminoncol.2019.01.003
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The approval by the Institutional Review Board is not applicable because the study is a review of the literature.
Informed consent
There were no individual’s person data requiring consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brabo, E.P., Moraes, A.B. & Neto, L.V. The role of immune checkpoint inhibitor therapy in advanced adrenocortical carcinoma revisited: review of literature. J Endocrinol Invest 43, 1531–1542 (2020). https://doi.org/10.1007/s40618-020-01306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01306-5