Abstract
Purpose
The dual antiproliferative mechanism of mycophenolate appears to be beneficial in Graves’ orbitopathy (GO).
Methods
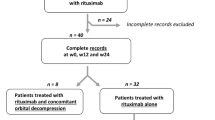
Safety data from the two published mycophenolate trials and the original database of the European Group on Graves’ Orbitopathy (EUGOGO) trial were systematically analyzed. Treatment efficacy stratified by individual visual parameters of activity and severity were compared.
Results
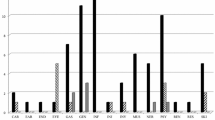
A total of 129 adverse events (AE) involving 50 patients (29.4%) were noted among all mycophenolate-treated patients. Mycophenolate sodium plus intravenous glucocorticoid (MPS + GC) group of the EUGOGO trial recorded significantly more AE (55.4% versus 4.6% of patients affected) and serious adverse events (SAE) (12.5% versus 0%) than mycophenolate mofetil (MMF) group of the Chinese trial. None of those SAE was side effect (SE). Most SE in MPS + GC group were mild. Gastrointestinal disorders, infection and liver dysfunction affected 8.8%, 7.1% and 1.2% of all mycophenolate-treated patients (versus 5.4%, 5.4% and 1.2% of all patients on GC monotherapy, respectively). MPS + GC did not significantly increase the risk of infection or liver dysfunction when compared to GC monotherapy. No cytopenia, serious infection or treatment-related mortality was reported. The much higher AE rates of mycophenolate trials in other autoimmune diseases or transplantations suggested that major mycophenolate toxicities were mostly dose- and duration dependent. Mycophenolate, either as monotherapy or as combination, achieved better overall response than GC monotherapy.
Conclusion
The risk–benefit ratio of low-dose mycophenolate treatment in active moderate-to-severe GO is highly favorable given its reassuring safety profile with low rate of mild-to-moderate SE and promising efficacy.



Similar content being viewed by others
References
Bartalena L, Fatourechi V (2014) Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 37(8):691–700
Hai YP, Lee ACH, Frommer L, Diana T, Kahaly GJ (2019) Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J Endocrinol Invest 19:15
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36(6):444–449
Bartalena L, Macchia PE, Marcocci C, Salvi M, Vermiglio F (2015) Effects of treatment modalities for Graves’ hyperthyroidism on Graves’ orbitopathy: a 2015 Italian Society of Endocrinology Consensus Statement. J Endocrinol Invest 38(4):481–487
Staatz CE, Tett SE (2014) Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol 88(7):1351–1389
Allison AC, Eugui EM (2000) Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47(2–3):85–118
Eugui EM, Allison AC (1993) Immunosuppressive activity of mycophenolate mofetil. Ann N Y Acad Sci 685:309–329
Budde K, Durr M, Liefeldt L, Neumayer HH, Glander P (2010) Enteric-coated mycophenolate sodium. Expert Opin Drug Saf 9(6):981–994
Wang J, Wang YT, Shao JQ, Wang X, Du H (2004) Immunosuppressive therapies in patients with Graves’ ophthalmopathy. Zhonghua Nei Ke Za Zhi 43(2):125–127
Riedl M, Kuhn A, Kramer I, Kolbe E, Kahaly GJ (2016) Prospective, systematically recorded mycophenolate safety data in Graves’ orbitopathy. J Endocrinol Invest 39(6):687–694
Ye X, Bo X, Hu X, Cui H, Lu B, Shao J et al (2017) Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clin Endocrinol (Oxf) 86(2):247–255
Kahaly GJ, Riedl M, Konig J, Pitz S, Ponto K, Diana T et al (2018) Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 6(4):287–298
Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL et al (2016) 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26(10):1343–1421
Tsai CC, Kau HC, Kao SC, Hsu WM (2006) Exophthalmos of patients with Graves’ disease in Chinese of Taiwan. Eye (Lond) 20(5):569–573
de Juan E Jr., Hurley DP, Sapira JD (1980) Racial differences in normal values of proptosis. Arch Intern Med 140(9):1230–1231
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10(6):521
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14(5):403–406
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S et al (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14(8):631–634
Moleti M, Giuffrida G, Sturniolo G, Squadrito G, Campenni A, Morelli S et al (2016) Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy. Endocrine 54(1):259–268
Rerolle JP, Szelag JC, Le Meur Y (2007) Unexpected rate of severe leucopenia with the association of mycophenolate mofetil and valganciclovir in kidney transplant recipients. Nephrol Dial Transplant 22(2):671–672
Mendes MM, Carminatti M, Pinheiro HS (2017) Severe sepsis from a Ciprofloxacin resistant salmonellosis in a kidney transplant recipient. J Bras Nefrol 39(1):82–85
Ordi-Ros J, Saez-Comet L, Perez-Conesa M, Vidal X, Mitjavila F, Castro Salomo A et al (2017) Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 76(9):1575–1582
Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E et al (2014) A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology 121(10):1863–1870
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC et al (2016) Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 4(9):708–719
Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D et al (2017) Mycophenolate mofetil combined with prednisone versus full-dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. Am J Kidney Dis 69(6):788–795
Remy P, Audard V, Natella PA, Pelle G, Dussol B, Leray-Moragues H et al (2018) An open-label randomized controlled trial of low-dose corticosteroid plus enteric-coated mycophenolate sodium versus standard corticosteroid treatment for minimal change nephrotic syndrome in adults (MSN Study). Kidney Int 94(6):1217–1226
Yunyun F, Yu P, Panpan Z, Xia Z, Linyi P, Jiaxin Z et al (2019) Efficacy and safety of low dose Mycophenolate mofetil treatment for immunoglobulin G4-related disease: a randomized clinical trial. Rheumatology (Oxf) 58(1):52–60
Tuin J, Stassen PM, Bogdan DI, Broekroelofs J, van Paassen P, Cohen Tervaert JW et al (2019) Mycophenolate mofetil versus cyclophosphamide for the induction of remission in nonlife-threatening relapses of antineutrophil cytoplasmic antibody-associated vasculitis: randomized, controlled trial. Clin J Am Soc Nephrol 14(7):1021–1028
Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R et al (2005) Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant 24(5):517–525
Strueber M, Warnecke G, Fuge J, Simon AR, Zhang R, Welte T et al (2016) Everolimus versus mycophenolate mofetil de novo after lung transplantation: a prospective, randomized, open-label trial. Am J Transplant 16(11):3171–3180
Qazi Y, Shaffer D, Kaplan B, Kim DY, Luan FL, Peddi VR et al (2017) Efficacy and safety of everolimus plus low-dose tacrolimus versus mycophenolate mofetil plus standard-dose tacrolimus in de novo renal transplant recipients: 12-month data. Am J Transplant 17(5):1358–1369
Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN et al (2019) 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 78(6):736–745
Bartley GB (2011) Rundle and his curve. Arch Ophthalmol 129(3):356–358
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C et al (2016) The 2016 European thyroid association/european group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J. 5(1):9–26
Perros P, Crombie AL, Matthews JN, Kendall-Taylor P (1993) Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol (Oxf) 38(4):367–372
Wiersinga WM (2013) Smoking and thyroid. Clin Endocrinol (Oxf) 79(2):145–151
Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker FW, Wiersinga WM (2005) Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 62(2):145–155
Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ (2010) A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab 95(5):2123–2131
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ (2011) Clinical relevance of thyroid-stimulating immunoglobulins in Graves’ ophthalmopathy. Ophthalmology 118(11):2279–2285
Ponto KA, Diana T, Binder H, Matheis N, Pitz S, Pfeiffer N et al (2015) Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J Endocrinol Invest 38(7):769–777
Kampmann E, Diana T, Kanitz M, Hoppe D, Kahaly GJ (2015) Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: a prospective study. Int J Endocrinol 2015:678194
Kahaly GJ (2015) Bioassays for TSH receptor antibodies: quo vadis? Eur Thyroid J. 4(1):3–5
Diana T, Wuster C, Olivo PD, Unterrainer A, Konig J, Kanitz M et al (2017) Performance and specificity of 6 immunoassays for TSH receptor antibodies: a multicenter study. Eur Thyroid J. 6(5):243–249
Kahaly GJ, Wuster C, Olivo PD, Diana T (2019) High titers of thyrotropin receptor antibodies are associated with orbitopathy in patients with Graves disease. J Clin Endocrinol Metab 104(7):2561–2568
Author information
Authors and Affiliations
Contributions
Literature search and data interpretation were performed by Riedl and Lee. The manuscript was written by Lee, Riedl and Kahaly. Kahaly, Diana and Frommer critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors stated that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, A.C.H., Riedl, M., Frommer, L. et al. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Invest 43, 767–777 (2020). https://doi.org/10.1007/s40618-019-01161-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01161-z