Abstract
Purpose
The clinical utility of rituximab (RTX) in Graves’ orbitopathy (GO) treatment remains controversial since the discrepant results from 2 prospective randomized studies (Stan M et al. J Clin Endocrinol Metab 2015; Salvi M et al. J Clin Endocrinol Metab 2015).
The aim of this study was to assess in real life the characteristics and the clinical outcomes of patients with GO treated with RTX in cases of corticosteroid resistance or corticosteroid dependence.
Methods
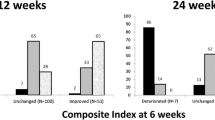
Multicenter French retrospective study including patients with moderate-to-severe GO requiring second-line treatment with RTX. Patients were classified according to three main baseline characteristics: clinical inflammation (CAS ≥ 3), oculomotor limitation, and visual dysfunction. Patients were considered as responders if, at 24 weeks (week 24), at least 1 of these 3 parameters improved with no worsening elsewhere.
Results
Forty patients were included (65% smokers, 38% dysthyroidism). Thirty-two patients were treated with RTX alone (one patient excluded owing to side effects): 64.5% had favorable responses at week 24 and significant reduction in baseline CAS (3.29 ± 1.6) at 12 weeks (1.93 ± 1.1; P < 0.001) and at week 24 (1.59 ± 1.1; P < 0.001); reduction in anti-TSH receptor antibodies at week 24 (P < 0.01); and significant improvement of visual acuity (P = 0.04) and ocular hypertonia (P = 0.04) at week 12, but no improvement in oculomotor dysfunction. Eight patients needed emergency treatment with concomitant RTX and orbital decompression, with favorable outcome for 5 patients. Predictive factors for a poor response to RTX were low baseline CAS, smoker, and baseline ocular hypertonia. All patients reported good tolerance except one serious side effect (a cytokine release syndrome).
Conclusions
The efficiency results of RTX in reducing CAS in this cohort are just between those of Stan and Salvi. This could be explained by our delay before treatment initiation, quicker than Stan but longer than Salvi. RTX appears to be effective as a second-line treatment for the inflammatory component of GO, especially if the disease is highly active and recent.





Similar content being viewed by others
References
Bartalena L, Baldeschi L, Boboridis K et al (2016) The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur Thyroid J 5:9–26. https://doi.org/10.1159/000443828
Kahaly GJ, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240. https://doi.org/10.1210/jc.2005-0148
Le Moli R, Baldeschi L, Saeed P et al (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in graves’ ophthalmopathy. Thyroid 17:357–362. https://doi.org/10.1089/thy.2006.0267
El Fassi D, Nielsen CH, Bonnema SJ et al (2007) B lymphocyte depletion with the monoclonal antibody rituximab in graves’ disease: a controlled pilot study. J Clin Endocrinol Metab 92:1769–1772. https://doi.org/10.1210/jc.2006-2388
Khanna D, Chong KKL, Afifiyan NF et al (2010) Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology 117:133–139.e2. https://doi.org/10.1016/j.ophtha.2009.05.029
Mitchell AL, Gan EH, Morris M et al (2013) The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory graves’ orbitopathy. Clin Endocrinol 79:437–442. https://doi.org/10.1111/cen.12141
Silkiss RZ, Reier A, Coleman M, Lauer SA (2010) Rituximab for thyroid eye disease. Ophthal Plast Reconstr Surg 26:310–314. https://doi.org/10.1097/IOP.0b013e3181c4dfde
Suhler EB, Lim LL, Beardsley RM et al (2014) Rituximab therapy for refractory orbital inflammation: results of a phase I/II dose-ranging randomized clinical trial. JAMA Ophthalmol 132:572–578. https://doi.org/10.1001/jamaophthalmol.2013.8179
Madaschi S, Rossini A, Formenti I et al (2010) Treatment of thyroid-associated orbitopathy with rituximab--a novel therapy for an old disease: case report and literature review. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 16:677–685. https://doi.org/10.4158/EP09385.RA
Şimşek T, Yıldırım N, Efe B, Kebapçı N (2017) Rituximab treatment in a patient with active graves’ Orbitopathy and psoriasis. Turk J Ophthalmol 47:42–46. https://doi.org/10.4274/tjo.26780
Stan MN, Garrity JA, Carranza Leon BG et al (2015) Randomized controlled trial of rituximab in patients with graves’ orbitopathy. J Clin Endocrinol Metab 100:432–441. https://doi.org/10.1210/jc.2014-2572
Salvi M, Vannucchi G, Currò N et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100:422–431. https://doi.org/10.1210/jc.2014-3014
Stan MN, Salvi M (2017) MANAGEMENT OF ENDOCRINE DISEASE: rituximab therapy for graves’ orbitopathy - lessons from randomized control trials. Eur J Endocrinol 176:R101–R109. https://doi.org/10.1530/EJE-16-0552
Perros P, Crombie AL, Matthews JN, Kendall-Taylor P (1993) Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol 38:367–372
Prummel MF, Wiersinga WM (1993) Smoking and risk of graves’ disease. JAMA 269:479–482
Prummel MF, Wiersinga WM, Mouritis MP, Koornneef L, Berghout A, van der Gaag R (1989) Amelioration of eye changes of graves’ ophthalmopathy by achieving euthyroidism. Acta Endocrinol 21(Suppl 2):185–189
Bartalena L, Marcocci C, Tanda ML et al (1998) Cigarette smoking and treatment outcomes in graves ophthalmopathy. Ann Intern Med 129:632–635
Vannucchi G, Campi I, Bonomi M et al (2010) Rituximab treatment in patients with active graves’ orbitopathy: effects on proinflammatory and humoral immune reactions. Clin Exp Immunol 161:436–443. https://doi.org/10.1111/j.1365-2249.2010.04191.x
Salvi M, Vannucchi G, Currò N et al (2012) Small dose of rituximab for graves orbitopathy: new insights into the mechanism of action. Arch Ophthalmol Chic Ill 1960 130:122–124. https://doi.org/10.1001/archopthalmol.2011.1215
Acknowledgments
We gratefully acknowledge Brigitte Dessome (Nantes University Hospital) and Matthieu Wargny (L’institut du thorax, CIC Endocrinology, and Inserm UMR 1087) for their helpful advice in statistical analysis. We thank Dr. Sophie Arsène, Dr. Peggy Pierre, Dr. Laurence Leenhardt, Dr. Claire Bournaud, Dr. Claire Briet, Dr. Bernard Goichot, Dr. Isabelle Raingeard, Dr. Nathalie Roudaut, Dr. Matthieu Pichelin, Dr. Ester Landau, Dr. Maëlle Le Bras, and Dr. Bertrand Vabres for providing us with their clinical data. We also thank the members of the French Thyroid Research Group (GRT).
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed were in accordance with the 1964 Helsinki Declaration and its later amendments.
The study protocol was accepted by the local ethics committee (GNEDS: “Groupe Nantais d’Ethique dans le Domaine de la Santé”). Informed consent was not required for this non-interventional retrospective study.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2933 kb)
Rights and permissions
About this article
Cite this article
Deltour, JB., d’Assigny Flamen, M., Ladsous, M. et al. Efficacy of rituximab in patients with Graves’ orbitopathy: a retrospective multicenter nationwide study. Graefes Arch Clin Exp Ophthalmol 258, 2013–2021 (2020). https://doi.org/10.1007/s00417-020-04651-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04651-6