Abstract
Introduction and aim
A prompt diagnosis of Cushing’s Syndrome (CS) in high-risk populations is mandatory: 1-mg dexamethasone suppression test (1-mg DST), late night salivary cortisol (LNSC), and urinary-free cortisol (UFC) are recommended, despite thresholds calculated in retrospective studies. Our aim was to study the diagnostic accuracy of LNSC measured with chemiluminescence assay in a prospective study, confirming discrepancies with mass spectrometry (MS).
Materials and methods
We enrolled 117 controls and 164 suspected CS (CS = 47, non-CS = 117). In case of increased LNSC, high clinical suspicion of CS or adrenal incidentaloma, patients were hospitalized to exclude/confirm CS.
Results
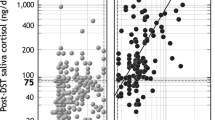
LNSC levels were higher in patients with suspected CS, CS, and non-CS than controls. Considering 16 nmol/L as threshold for CS, overall LNSC revealed SE 97% and SP 84% in the whole group of subjects considered, achieving positive/negative likelihood ratio of 5.56/0.045, respectively. 35 out of 81 subjects with increased LNSC were non-CS (15 diabetic and 20 obese): considering only those patients with increased likelihood to have a CS (the non-CS patients) SP decreased to 70%, and further reduced to 60% if we discharged subjects with adrenal incidentaloma. MS analyses reduced partially the number of false-positive LNSC.
Conclusions
LNSC measured in automated chemiluminescence is reliable in clinical practice: it present a high diagnostic accuracy to exclude hypercortisolism in patients with normal cortisol levels. MS could be used to reduce the number of false-positive results; nevertheless, some non-CS subjects with functional hypercortisolism could have a mild impairment of cortisol rhythm.



Similar content being viewed by others
References
Clayton RN, Raskauskiene D, Reulen RC, Jones PW (2011) Mortality and morbidity in Cushing’s Disease over 50 years in Stoke-On-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab 96:632–642
Dekkers OM, Horváth-Puhó E, Jørgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, Pereira AM, Sørensen HT (2013) Multisystem morbidity and mortality in cushing’s syndrome: a cohort study. J Clin Endocrinol Metab 98:2277–2284
Boscaro M, Arnaldi G (2009) Approach to the patient with possible cushing’s syndrome. J Clin Endocrinol Metab 94:3121–3131
Tirabassi G, Boscaro M, Arnaldi G (2014) Harmful effects of functional hypercortisolism: a working hypothesis. Endocrine 46:370–386
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s Syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540
Raff H (2012) Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary. 15:64–70
Ceccato F, Barbot M, Zilio M, Ferasin S, Occhi G, Daniele A, Mazzocut S, Iacobone M, Betterle C, Mantero F, Scaroni C (2013) Performance of salivary cortisol in the diagnosis of cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol 169:31–36
Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK (2007) Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab 92:3102–3107
Belaya ZE, Iljin AV, Melnichenko GA, Rozhinskaya LY, Dragunova NV, Dzeranova LK, Butrova SA, Troshina EA, Dedov II (2012) Diagnostic performance of late-night salivary cortisol measured by automated electrochemiluminescence immunoassay in obese and overweight patients referred to exclude cushing’s syndrome. Endocrine 41:450–494
Carrasco CA, García M, Goycoolea M, Cerda J, Bertherat J, Padilla O, Meza D, Wohllk N, Quiroga T (2012) Reproducibility and performance of one or two samples of salivary cortisol in the diagnosis of Cushing’s Syndrome using an automated immunoassay system. Endocrine 41:487–493
Nunes M, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A (2009) Late-night salivary cortisol for diagnosis of overt and subclinical cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94:456–462
Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M (2015) Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in Cushing’s Syndrome. Clin Chim Acta 451:247–251
Erickson D, Singh RJ, Sathananthan A, Vella A, Bryant SC (2012) Late-night salivary cortisol for diagnosis of cushing’s syndrome by liquid chromatography/tandem mass spectrometry assay. Clin Endocrinol 76:467–472
Trementino L, Zilio M, Marcelli G, Michetti G, Barbot M, Ceccato F, Boscaro M, Scaroni C, Arnaldi G (2015) The role of an acute pasireotide suppression test in predicting response to treatment in patients with Cushing’s Disease: findings from a pilot study. Endocrine 50:154–161
Trementino L, Cardinaletti M, Concettoni C, Marcelli G, Polenta B, Spinello M, Boscaro M, Arnaldi G (2015) Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing’s Disease. Pituitary. 18:60–67
Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, Antonelli G, Plebani M, Mantero F, Boscaro M, Scaroni C (2015) Screening tests for Cushing’s Syndrome: urinary free cortisol role measured by LC–MS/MS. J Clin Endocrinol Metab 100:3856–3861
Simel DL, Samsa GP, Matchar DB (1991) Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 44:763–770
Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, Mann K, Petersenn S (2012) Salivary cortisol as a diagnostic tool for Cushing’s Syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol 166:613–618
Manetti L, Rossi G, Grasso L, Raffaelli V, Scattina I, Del Sarto S, Cosottini M, Iannelli A, Gasperi M, Bogazzi F, Martino E (2013) Usefulness of salivary cortisol in the diagnosis of hypercortisolism: comparison with serum and urinary cortisol. Eur J Endocrinol 168:315–321
Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM (2008) Accuracy of diagnostic tests for cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 93:1553–1562
Graham UM, Hunter SJ, McDonnell M, Mullan KR, Atkinson AB (2013) A comparison of the use of urinary cortisol to creatinine ratios and nocturnal salivary cortisol in the evaluation of cyclicity in patients with cushing’s syndrome. J Clin Endocrinol Metab 98:E76–E76
Carrozza C, Corsello SM, Paragliola RM, Ingraudo F, Palumbo S, Locantore P, Sferrazza A, Pontecorvi A, Zuppi C (2010) Clinical accuracy of midnight salivary cortisol measured by automated electrochemiluminescence immunoassay method in Cushing’s Syndrome. Ann Clin Biochem 47:228–232
Repetto EM, Gonzalez D, Jacobsen D, Smithuis F, Jamardo J, Cano M, Aranda C, Oneto A, Berg G, Fabre B (2017) Evaluation of an automated chemiluminescent immunoassay for salivary cortisol measurement. Utility in the diagnosis of Cushing’s syndrome. Clin Chem Lab Med 1:e65–e68. https://doi.org/10.1515/cclm-2016-0585
Ceccato F, Barbot M, Zilio M, Ferasin S, De Lazzari P, Lizzul L, Boscaro M, Scaroni C (2015) Age and the metabolic syndrome affect salivary cortisol rhythm: data from a community sample. Hormones 14:392–398
Coelli S, Farias CB, Soares AA, Crescente GM, Hirakata VN, Souza LB, Czepielewski MA, Camargo JL, Silveiro SP (2017) Influence of age, gender and body mass index on late-night salivary cortisol in healthy adults. Clin Chem Lab Med 55:1954–1961
Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I (2009) The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol 160:87–92
Palmieri S, Morelli V, Polledri E, Fustinoni S, Mercadante R, Olgiati L, Eller Vainicher C, Cairoli E, Zhukouskaya VV, Beck-Peccoz P, Chiodini I (2013) The role of salivary cortisol measured by liquid chromatography-tandem mass spectrometry in the diagnosis of subclinical hypercortisolism. Eur J Endocrinol 168:289–296
Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA (2014) Differentiating between Cushing’s disease and Pseudo-Cushing’s Syndrome: comparison of four tests. Eur J Endocrinol 170:477–486
Newell-Price J, Trainer P, Besser M, Grossman A (1998) The diagnosis and differential diagnosis of cushing’s syndrome and pseudo-cushing’s states. Endocr Rev 19:647–672
Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F (2007) Specificity of first-line tests for the diagnosis of cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab 92:4123–4129
Reimondo G, Allasino B, Bovio S, Paccotti P, Angeli A, Terzolo M (2005) Evaluation of the effectiveness of midnight serum cortisol in the diagnostic procedures for cushing’s syndrome. Eur J Endocrinol 153:803–809
Papanicolaou DA, Yanovski JA, Cutler GB, Chrousos GP, Nieman LK (1998) A single midnight serum cortisol measurement distinguishes cushing’s syndrome from pseudo-cushing States1. J Clin Endocrinol Metab 83:1163–1167
Ceccato F, Antonelli G, Frigo AC, Regazzo D, Plebani M, Boscaro M, Scaroni C (2017) First-line screening tests for cushing’s syndrome in patients with adrenal incidentaloma: the role of urinary free cortisol measured by LC–MS/MS. J Endocrinol Invest 40:753–760
Sereg M, Tőke J, Patócs A, Varga I, Igaz P, Szücs N, Horányi J, Pusztai P, Czirják S, Gláz E, Rácz K, Tóth M (2011) Diagnostic performance of salivary cortisol and serum osteocalcin measurements in patients with overt and subclinical cushing’s syndrome. Steroids 76:38–42
Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J (2014) Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab 99:4462–4470
Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R (2014) Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol 2:396–405
Ceccato F, Antonelli G, Frigo AC, Regazzo D, Plebani M, Boscaro M, Scaroni C (2017) First-line screening tests for Cushing’s syndrome in patients with adrenal incidentaloma: the role of urinary free cortisol measured by LC-MS/MS. J Endocrinol Invest 40:753–760
Zerikly RK, Amiri L, Faiman C, Gupta M, Singh RJ, Nutter B, Kennedy L, Hatipoglu B, Weil RJ, Hamrahian AH (2010) Diagnostic characteristics of late-night salivary cortisol using liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 95:4555–4559
Cardoso EM, Arregger AL, Tumilasci OR, Contreras LN (2009) Diagnostic value of salivary cortisol in Cushing’s syndrome (CS). Clin Endocrinol 70:516–521
Acknowledgements
Authors are grateful to prof. Carla Scaroni for the critical review of the manuscript.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest that might be perceived as influencing the impartiality of the reported research.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
F. Ceccato and G. Marcelli should be considered jointly as first co-authors.
Rights and permissions
About this article
Cite this article
Ceccato, F., Marcelli, G., Martino, M. et al. The diagnostic accuracy of increased late night salivary cortisol for Cushing’s syndrome: a real-life prospective study. J Endocrinol Invest 42, 327–335 (2019). https://doi.org/10.1007/s40618-018-0921-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0921-1