Abstract
Purpose
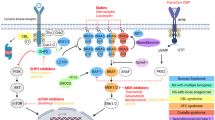
Gitelman’s syndrome (GS) presents normo-hypotension and absence of cardiovascular–renal remodeling despite high angiotensin II (Ang II), activation of renin–angiotensin–aldosterone system and is a human model of endogenous antagonism of Ang II signaling, opposite to hypertension. GS’s clinical presentation leads to questions regarding what features might be responsible. One area of investigation involves Ang II signaling. In hypertensive patients, RhoA/Rho kinase (RhoA/ROCK) pathway activation by Ang II is involved in hypertension development/maintenance and induction of long-term consequences (cardiovascular–renal remodeling), while GS has reduced p63RhoGEF gene and protein levels and ROCK activity. Ang II signaling is mediated by Gαq, which interacts with p63RhoGEF via the α6–αN linker connecting p63RhoGEF’s DH and PH domains acting as a conformational switch to activate RhoA/ROCK signaling.
Methods
We have investigated in GS patients, the presence of mutations in either p63RhoGEF’s α6–αN linker domain and in Gαq’s Ala253, Trp263, and Tyr356 residues, crucial for p63RhoGEF–Gαq interplay.
Results
No mutations have been found in specific aminoacids of p63RhoGEF α6–αN linker and Gαq, key for p63RhoGEF/Gαq interplay.
Conclusions
Gitelman’s syndrome normo/hypotension and lack of cardiovascular–renal remodeling are not due to mutations of p63RhoGEF α6–αN linker and Gαq interactions. This opens the way for investigations on different coding and no-coding regions (p63RhoGEF and Gαq promoters) and on altered transcriptional/post-transcriptional regulation. Clarification of how these biochemical/molecular mechanisms work/interact would provide insights into mechanisms involved in the GS’s Ang II signaling fine tuning, in human physiology/pathophysiology in general and could also identify significant targets for intervention in the treatments of hypertension.


Similar content being viewed by others
References
Calo LA, Davis PA, Rossi GP (2014) Understanding the mechanisms of angiotensin II signaling involved in hypertension and its long-term sequelae: insights from Bartter’s and Gitelman’s syndromes, human models of endogenous angiotensin II signaling antagonism. J Hypertens 32:2109–2119. doi:10.1097/HJH.0000000000000321 (discussion 2119)
Calo LA, Davis PA, Pagnin E, Dal Maso L, Maiolino G, Seccia TM, Pessina AC, Rossi GP (2014) Increased level of p63RhoGEF and RhoA/Rho kinase activity in hypertensive patients. J Hypertens 32:331–338. doi:10.1097/HJH.0000000000000075
Cai A, Li L, Zhou Y (2016) Pathophysiological effects of RhoA and Rho-associated kinase on cardiovascular system. J Hypertens 34:3–10. doi:10.1097/HJH.0000000000000768
Loirand G, Guerin P, Pacaud P (2006) Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 98:322–334 (doi:98/3/322)
Loirand G, Pacaud P (2010) The role of Rho protein signaling in hypertension. Nat Rev Cardiol 7:637–647. doi:10.1038/nrcardio.2010.136
Ravarotto V, Pagnin E, Maiolino G, Fragasso A, Carraro G, Rossi B, Calo LA (2015) The blocking of angiotensin II type 1 receptor and RhoA/Rho kinase activity in hypertensive patients: effect of olmesartan medoxomil and implication with cardiovascular-renal remodeling. J Renin Angiotensin Aldosterone Syst 16:1245–1250. doi:10.1177/1470320315594324
Sanchez-Fernandez G, Cabezudo S, Garcia-Hoz C, Beninca C, Aragay AM, Mayor F Jr, Ribas C (2014) Galphaq signalling: the new and the old. Cell Signal 26:833–848. doi:10.1016/j.cellsig.2014.01.010
Berridge MJ (1993) Inositol trisphosphate and calcium signalling. Nature 361:315–325. doi:10.1038/361315a0
Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T (2005) The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. J Biol Chem 280:11134–11139 (doi:M411322200)
Momotani K, Somlyo AV (2012) p63RhoGEF: a new switch for G(q)-mediated activation of smooth muscle. Trends Cardiovasc Med 22:122–127. doi:10.1016/j.tcm.2012.07.007
Momotani K, Artamonov MV, Utepbergenov D, Derewenda U, Derewenda ZS, Somlyo AV (2011) p63RhoGEF couples Galpha(q/11)-mediated signaling to Ca2+ sensitization of vascular smooth muscle contractility. Circ Res 109:993–1002. doi:10.1161/CIRCRESAHA.111.248898
Shankaranarayanan A, Boguth CA, Lutz S, Vettel C, Uhlemann F, Aittaleb M, Wieland T, Tesmer JJ (2010) Galpha q allosterically activates and relieves autoinhibition of p63RhoGEF. Cell Signal 22:1114–1123. doi:10.1016/j.cellsig.2010.03.006
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Calo LA, Pessina AC (2007) RhoA/Rho-kinase pathway: much more than just a modulation of vascular tone. Evidence from studies in humans. J Hypertens 25:259–264. doi:10.1097/HJH.0b013e328010d4d2
Gabrielli L, Winter JL, Godoy I, McNab P, Padilla I, Cordova S, Rigotti P, Novoa U, Mora I, Garcia L, Ocaranza MP, Jalil JE (2014) Increased rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens 27:838–845. doi:10.1093/ajh/hpt234
Calo LA, Vertolli U, Pagnin E, Ravarotto V, Davis PA, Lupia M, Naso E, Maiolino G, Naso A (2016) Increased rho kinase activity in mononuclear cells of dialysis and stage 3-4 chronic kidney disease patients with left ventricular hypertrophy: cardiovascular risk implications. Life Sci 148:80–85. doi:10.1016/j.lfs.2016.02.019
Calo LA, Pagnin E, Davis PA, Sartori M, Ceolotto G, Pessina AC, Semplicini A (2004) Increased expression of regulator of G protein signaling-2 (RGS-2) in Bartter’s/Gitelman’s syndrome. A role in the control of vascular tone and implication for hypertension. J Clin Endocrinol Metab 89:4153–4157. doi:10.1210/jc.2004-0498
Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ (2003) Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest 111:1259
Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME (2003) Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med 9:1506–1512. doi:10.1038/nm958
Shankaranarayanan A, Thal DM, Tesmer VM, Roman DL, Neubig RR, Kozasa T, Tesmer JJ (2008) Assembly of high order G alpha q-effector complexes with RGS proteins. J Biol Chem 283:34923–34934. doi:10.1074/jbc.M805860200
Calo LA, Pagnin E, Ceolotto G, Davis PA, Schiavo S, Papparella I, Semplicini A, Pessina AC (2008) Silencing regulator of G protein signaling-2 (RGS-2) increases angiotensin II signaling: insights into hypertension from findings in Bartter’s/Gitelman’s syndromes. J Hypertens 26:938–945. doi:10.1097/HJH.0b013e3282f60d98
Semplicini A, Lenzini L, Sartori M, Papparella I, Calo LA, Pagnin E, Strapazzon G, Benna C, Costa R, Avogaro A, Ceolotto G, Pessina AC (2006) Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens 24:1115–1124. doi:10.1097/01.hjh.0000226202.80689.8f
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pagnin, E., Ravarotto, V., Maiolino, G. et al. Gαq/p63RhoGEF interaction in RhoA/Rho kinase signaling: investigation in Gitelman’s syndrome and implications with hypertension. J Endocrinol Invest 41, 351–356 (2018). https://doi.org/10.1007/s40618-017-0749-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-017-0749-0