Abstract
Background
The goal is to assess the usability and satisfaction of implementing the Getting2GoalSM protocol by physicians transitioning patients with type 2 diabetes (T2DM) from multiple daily injections (MDI) to continuous subcutaneous insulin infusion (CSII).
Methods
T2DM patients from three diabetes clinics were switched from MDI to CSII. Physicians used the Getting2Goal type 2 pumping protocol to prescribe and manage insulin pump therapy for T2DM. Surveys were conducted in which the physicians rated their feedback related to acceptability of the Getting2Goal on a 5-point Likert scale.
Results
17 patients with T2DM were switched from MDI to CSII treatment. Mean (±standard deviation) age was 61.2 ± 7.7 (46–77) years, weight was 91.4 ± 21 (66–147) kg, BMI was 31.9 ± 7.6, A1C was 9.2 ± 1.4 % (7.2–12.3) and TDD on MDI was 109.1 ± 53.1 units. Surveys completed by physicians indicated Getting2Goal type 2 pumping protocol to be more efficient, time saving, and structured compared to their current processes. In addition, the primarily prescribed TDD on pump was 98.1 ± 50.0 units and the TDD at first download was 81.4 ± 36.4 units, representing a 25.4 % reduction in TDD At first download. The percentage of all blood glucose readings below 70 mg/dL was also very low.
Conclusions
The data indicate Getting2Goal materials as a standard approach that is simple and efficient to initiate pump therapy for T2DM. At the same time, it is safe and a useful tool for physicians that are starting to prescribe pump therapy for T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effective use of insulin pump therapy versus multiple daily injections (MDI) in insulin taking patients with type 2 diabetes (T2DM) has been recently demonstrated in a randomized controlled, interventional post-market study (OpT2mise) [1]. The clinical outcomes data obtained from this study showed that subjects on continuous subcutaneous insulin infusion (CSII) had a significantly greater reduction in glycated hemoglobin (A1C) than subjects on multiple daily injections (MDI) (p ≤ 0.0001), with 55 % of subjects on CSII achieving an A1C < 8 versus 28 % if subjects were on MDI. At 6 months, there was a 20 % less insulin usage in the CSII group versus the MDI group [1]. The underlying motivations, initial deployment, and routine use of insulin pump therapy are considerably different in type 1 diabetes (T1DM) and T2DM. Studies focusing on uncontrolled, high insulin daily dosage and MDI requiring patients (i.e., insulin resistant) showed better outcome for patients starting on CSII in comparison to their previous conventional MDI. These were mainly observational, randomized studies that have shed some light on the practice of CSII initiation in T2DM [2].
There are studies that have compared the potential benefits of CSII versus MDI. In one single-center longitudinal, retrospective observational French study, 102 poorly controlled T2DM patients (baseline A1C 9.3 %, 78 mmol/mol) demonstrated a 21.5 % A1C reduction following CSII initiation [3]. Another French single-center study with 51 obese T2DM patients (A1C 9.4 %, 79 mmol/mol) demonstrated similar improvements in glycemic control following CSII treatment but with a twofold increase in TDD [4]. An observational study from 31 French hospitals reported a 25 % reduction in insulin dose from pre-CSII baseline [5]. The number of basal rates per patient was also very limited in these studies [3–5]. Studies from the USA, India, and Israel have also demonstrated glycemic control benefits with no change in the TDD [6–10].
The need for accurately configuring bolus by carbohydrate content and pre-meal glucose levels adjustment seems to be of less importance in comparison with T1DM patients. The data provided by Bergenstal et al. [11] show that a simple dosing regimen of fixed mealtime boluses for T2DM patients—with no carbohydrate counting—allows for glycemic control comparable to that achieved with a more elaborate regimen. It may also be that in this patient population, high doses of prandial and basal insulin contribute to chronic hyperinsulinemia, minimizing the benefits of fine adjustments to prandial insulin boluses.
As patients with T2DM on CSII need less elaborate operation of the insulin pump, the focus of education should be on a simple approach to pump use with emphasis on technical issues as dexterity and autonomy assessing the presence of a significant cognitive or operative disability [12].
Methods
This was a physician user evaluation study. It was conducted at three community based diabetes clinics (Rishon Lezion, Israel, Hadera, Israel, Ashkelon, Israel) and 1 at a hospital-based clinic (Holon, Israel). They were using the Getting2Goal protocol with their new T2DM patients who were considered for CSII therapy. These patients switched from MDI to Paradigm Veo™ 754 insulin pump (Medtronic® MiniMed®, Inc., Northridge, CA) with download capabilities by CareLink® Therapy Management Software for Diabetes (Medtronic MiniMed, Inc., Northridge, CA). The study collected data retrospectively and the physicians feedback on the usability of the protocol.
The Getting2Goal type 2 pumping protocol is Medtronic’s stepwise approach to prescribing and managing insulin pump therapy for T2DM formulated by Bruce W. Bode MD, Ohad Cohen MD, Scott Lee MD and Yves Reznik MD as shown in the supplement.
The Getting2Goal concept is intended to formulate the current concepts and approaches to insulin pump therapy in patients with T2DM into a practical work flow and to aid in the transition from MDI to CSII therapy. The physicians and health care professionals for whom these worksheets and forms are intended for, either come from experienced centers with T1DM patients on CSII, assisting on adjustments to patients with T2DM, or those who are experienced with patients with T2DM and have less or no experience with pump therapy. The worksheets and forms also help to delineate between the clinical responsibility in prescribing doses and treatment concepts (e.g., fixed versus variable meal doses). It also helps technical and educational issues which are instrumental in functional divisions and in decreasing the workload on physicians and health care providers.
The work sheets and forms are organized according to the work flow:
-
1.
Patient selection: definition of patients, including clinical criteria, suitable for CSII with the Getting2Goal protocol.
-
2.
Pump initiation:
-
(a)
Defining TDD and calculating initial pump settings. Two potential methods are presented: one is based on previously known insulin dose and the other is based on patient’s weight. The method based on previously known insulin dose recommends a 0–20 % reduction in TDD from the previous MDI regimen to CSII according to patients control and compliance.
-
(b)
Defining glycemic goals (setting customized goals).
-
(c)
Check list for simplified technical education—basic pump functions (1–2 basal rate and fixed mealtime boluses).
-
(a)
-
3.
Ongoing diabetes management: follow-up chart and recommendation for fine tuning of pumps parameters and bolus recommendations and a quick guide for understanding relevant information from the CareLink software download.
Previous use of medications (includes hypoglycaemic drugs) are not collected at baseline. However, during the process of pump initiation, subjects are instructed to the use of insulin and other glucose-lowering medications. Subjects are not to take premix, intermediate, long-acting insulin injection the day of the pump start. In addition, adjustment of other glucose-lowering medications is to be made. These include considering: (1) stop of sulfonylureas and meglitinides and (2) continuing metformin and incretin mimetics, insulin sensitizers and other glucose-lowering agents. Once subject reaches goal, the subject is to consider discontinuing medications one at a time to determine if BG control can be maintained or discontinuing TZD if patient has edema or weight gain.
Surveys were conducted in which the physicians rated their feedback related to acceptability of the Getting2Goal on a 5-point Likert scale. The perceived value and benefits were evaluated by whether or not physicians agreed with certain statements. No attempt was made to validate the survey because of the study’s short duration and small study sample size.
The three items surveyed were:
-
1.
Comparison of the physicians’ current processes to the use of the Getting2Goal type 2 pumping protocol and forms.
-
2.
Perceived effect of the simple bolus protocol on patient’s outlook on CSII therapy.
-
3.
Overall impression of the Getting2Goal type 2 pumping protocol and forms and willingness to apply it with upcoming patients.
In addition, the physician’s preference of insulin dose and bolus plan was recorded.
Three key questions were evaluated when assessing the utilization of the worksheets:
-
1.
What was the adjustment in TDD recommended by the physician on switching to CSII?
-
2.
What was the difference between the dosage before switching and the dosage as recorded at the first 2 week download?
-
3.
What was the percentage of capillary glucose measurements below 70 mg/dL and over 180 mg/dL at first download?
IRB consent for the use retrospective data was obtained.
Results
17 patients with T2DM were switched from MDI to CSII treatment. Mean [±standard deviation (SD)] age was 61.2 ± 7.7 (46–77) years, weight was 91.4 ± 21 (66–147) kg, BMI was 31.9 ± 7.6, A1C was 9.2 ± 1.4 % (7.2–12.3) and TDD on MDI was 109.1 ± 53.1 units.
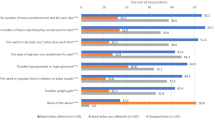
Surveys completed by the physician showed that the: Getting2Goal type 2 pumping protocol and forms was acceptable to be more efficient, time saving and structured in comparison to their current processes (Fig. 1). Most of the physicians preferred the simple approach (using a fixed insulin dose bolus plan with 1–2 basal rates). The physicians perceived that this approach made patients more willingly to accept recommendation of pump therapy, helped alleviate patient’s concern of the complexity of the transition, and increased patient’s information retention (Fig. 2).
Overall responses indicated that the physicians had a high level of satisfaction with the CareLink reports and would recommend to other physician the Getting2Goal program when transitioning T2DM patients into pump therapy (Fig. 3).
Insulin use
Pre-pump TDD use was 109.1 ± 53.1 units. The primarily prescribed TDD on pump was 98.1 ± 50.0 units and the TDD at first download was 81.4 ± 36.4 units, representing a 25.4 % reduction in TDD (Fig. 4). 47 % of patients were planned to stay on their MDI TDD, 47 % of patients were prescribed a 20 % reduction of TDD and 6 % were recommended a 30 % reduction. On the first download only, 20 % retained their pre-CSII TDD while 20, 27 and 33 % of patients had reduced their pre-CSII TDD by 20, 30 % and more than 30 %, respectively. No patients had either an increase in TDD as recommended at the initial pump setting or at first download (which occurred few weeks later with physician’s pump settings change).
Glycemic control
At first download, the average glucose was 167.8 mg/dL, average SD was 55.5 mg/dL, and both the number of blood glucose reading below 70 mg/dL and the percentage of all blood glucose readings below 70 mg/dL were very low (Table 1) with one patient contributing most of the glucose readings below 70 mg/dL (patient #1, Table 1).
Discussion
In this pilot survey, we attempted to assess physician’s acceptability of the Getting2Goal approach via the work sheets and forms from a response questioner, and to follow the implementation of the insulin plan as prescribed by the physician with the insulin pump download. The responses to the survey questionnaire were favorable as the physicians found the worksheets: useful and efficient in organizing the transition to CSII, assisting in patient management, saving time. The physicians were prepared to further use the worksheets in daily practice and to recommend the Getting2Goal approach to other physicians. Getting2Goal materials provide a standardized approach that is simple and efficient to initiating pump therapy. In addition, the CareLink software download is essential for T2DM as glycemic data are readily available for both physicians and patients.
The goal of optimization for insulin treatment was not intended for this study. For the first 2–4 weeks of T2DM subjects switching from MDI treatment to pump treatment, safety is of at most concern in this study. It was designed to assess that non-severe hypoglycaemia events are not increased and no decrease in glycemic control. Subjects’ insulin treatment was not intensified with standardized titration protocol to achieve preprandial and postprandial glycemic target ranges. Tweaking of insulin treatment for subjects was modified gradually as subjects continue.
The patients profile match the patients recruited to the OpT2mise randomized controlled study [1], and the profile of the patients in the studies that demonstrated advantage of CSII in T2DM [2, 3]. These are patients with baseline A1C above 8.5 % with high insulin dose per day and obesity.
Insulin dose were either unchanged or reduced by 20 % on initiation of CSII, while the doses at the first download, 2–4 weeks after CSII initiation, further decrease in TDD was noted, with only 20 % of the patients remaining on their pre-CSII doses. The reduction in insulin dose was associated with an average capillary blood glucose of 167.8 mg/dL, which is lower than the glucose equivalent of the baseline A1C of 9.2 % (220 mg/dL [13] with few measurements below 70 mg/dL). Although this cannot be used as any measure of efficacy, it does reveal that the use of the Getting2Goal guided initiation of CSII in these patients with T2DM was not associated with short-term adverse glycemic consequence.
This Getting2Goal was utilized in the OpT2mise study, a larger multi-national outcome study, which demonstrated solid evidence for greater A1C reduction with CSII when compared with MDI in type 2 diabetes patients on MDI. The protocol was not evaluated by HCP outside of the OpT2mise study so therefore only usability conclusions could be drawn from participating HCP. The results from the OpT2mise trial suggests that is of importance to select patients who could most benefit from pump therapy. The run-in period prior to randomization, the dose adjustment schedule, and the Getting2Goal guide for applying such adjustments, allowed the identification of patients who were potential candidate for pump therapy.
We acknowledged several limitations of the study: the geographical area, small sample size, retrospective nature, the absence of CGM and lack of percentage of values >70 and <180 mg/dL before starting protocol, and absence of an A1C value after protocol application.
These factors should be assessed in an appropriate study design in future studies to address if the protocol is an effective tool for improving glycemic control. However, the present study opens alternative options for those patients failing on current injection regimens, and suggests that pump therapy may therefore be considered a valuable therapeutic option in this population.
In conclusion, the use of the Getting2Goal worksheets are convenient and safe for use in transitioning patients with T2DM from MDI to insulin pump therapy and can be now assessed on a wider and multi-national level.
References
Reznik Y, Cohen O, Aronson R, Conget I, Runzis S, Castaneda J et al (2014) Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 384:1265–1272
Reznik Y, Cohen O (2013) Insulin pump for type 2 diabetes: use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care 36(2):S219–S225
Reznik Y, Morera J, Rod A et al (2010) Efficacy of continuous subcutaneous insulin infusion in type 2 diabetes mellitus: a survey on a cohort of 102 patients with prolonged follow-up. Diabetes Technol Ther 12(12):931–936
Charras L, Sanz C, Labrousse-Lhermine F, Cazals L, Hanaire H (2012) Traitement par pompe à insuline dans le diabète de type 2 (DT2): 12 ans de suivi d’une cohorte de 50 patients (Abstract). Diabetes Metab 38:A11 (in French)
Courrèges JP, Donnet JP, Gouet D et al (2012) Résultats métaboliques obtenus à 2 ans sous pompe à insuline ambulatoire chez des diabétiques de type 2 en échec d’insulinothérapie optimisée (Abstract). Diabetes Metab 38:A98 (in French)
HermanWH Ilag LL, Johnson SL et al (2005) A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care 28(7):1568–1573
Berthe E, Lireux B, Coffin C et al (2007) Effectiveness of intensive insulin therapy by multiple daily injections and continuous subcutaneous infusion: a comparison study in type 2 diabetes with conventional insulin regimen failure. Horm Metab Res 39(3):224–229
Wainstein J, Metzger M, Boaz M et al (2005) Insulin pump therapy vs. multiple daily injections in obese type 2 diabetic patients. Diabet Med 22(8):1037–1046
Edelman SV, Bode BW, Bailey TS et al (2010) Insulin pump therapy in patients with type 2 diabetes safely improved glycemic control using a simple insulin dosing regimen. Diabetes Technol Ther 12(8):627–633
Kesavadev J, Balakrishnan S, Ahammed S, Jothydev S (2009) Reduction of glycosylated hemoglobin following 6 months of continuous subcutaneous insulin infusion in an Indian population with type 2 diabetes. Diabetes Technol Ther 11(8):517–521
Bergenstal RM, Johnson M, Powers MA et al (2008) Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 31(7):1305–1310
Lassmann-Vague V, Clavel S, Guerci B et al (2010) Société francophone du diabète (ex ALFEDIAM). When to treat a diabetic patient using an external insulin pump. Expert consensus. Société francophone du diabète (ex ALFEDIAM) 2009. Diabetes Metab 36(1):79–85
Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D et al (2008) Translating the A1C assay into estimated average glucose values. Diabetes Care 31:1473–1478
Acknowledgments
The authors received editorial/writing support in the preparation of this manuscript provided by Xuan Nguyen, funded by Medtronic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ohad Cohen has received research grants, travel support, and consultancy fees from Sanofi. In addition, O. Cohen is also employed by Medtronic, Inc., as Medical Affairs Director, Diabetes (Israel). Medtronic, Eli Lilly. Zuhdi Agabria, Lyudmila Lysyy, Yuri Ianovitsky have no disclosures in regard to this publication. Julio Wainstein received study grants from Medtronic. Xuan Nguyen, Scott Lee and Michele Fung are employees of Medtronic. Other than above the authors declare they have no conflict of interest.
Ethical approval
The study was conducted in compliance with Ethical Standards and IRB approval.
Informed consent
As the data was anonymised there was no contact with the patients, and the IRB approved the use of all individual data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cohen, O., Agabria, Z., Lysyy, L. et al. Adaptability of structured forms for CSII initiation in patients with type 2 diabetes the Getting2GoalSM concept. J Endocrinol Invest 39, 627–633 (2016). https://doi.org/10.1007/s40618-015-0407-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0407-3