Abstract
Purpose
Randomized trials show that liraglutide 1.8 mg is more effective than 1.2 mg in reducing HbA1c, but dose escalation is neither routinely considered nor recommended by some guidelines. We report real world data on the effects of dose-escalating liraglutide from 1.2 to 1.8 mg.
Methods
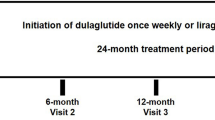
In a pseudo-prospective, case–control study, patients who underwent liraglutide dose escalation to 1.8 mg for not having met individualized targets while on the 1.2 mg dose (n = 52) were compared to matched patients who remained on 1.2 mg (n = 52) for having shown good response, as defined by the patient’s own diabetologist. HbA1c was recorded at ≤6-month intervals until the end of observation.
Results
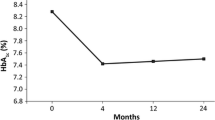
The two groups were matched for all clinical characteristics, including baseline HbA1c (8.5 %). During a 12-month follow-up, patients who remained on liraglutide 1.2 mg showed a maximal HbA1c reduction of 1.29 ± 0.15 %. Patients who escalated to 1.8 mg showed a lower HbA1c reduction during therapy with 1.2 mg than controls (0.58 ± 0.16 %; p = 0.0017). Escalation to 1.8 mg resulted in a further HbA1c reduction of 0.62 ± 0.17 %. During a total 18-month follow-up, patients who escalated to 1.8 mg showed a total maximal HbA1c reduction of 0.84 ± 0.22 %. At the end of the observation, HbA1c was 7.54 ± 0.17 % in patients who remained on 1.2 mg and 7.92 ± 0.21 in patients who escalated to 1.8 mg (p = 0.13). Escalation to 1.8 mg also helped further body weight reduction.
Conclusions
Escalating liraglutide dose to 1.8 mg in patients who responded less than expected to 1.2 mg helps in reducing HbA1c and reaching therapeutic targets.

Similar content being viewed by others
References
Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35(6):1364–1379. doi:10.2337/dc12-0413
Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thogersen H, Wilken M, Agerso H (2000) Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 43(9):1664–1669 (jm9909645)
European Medicines Agency Victoza 6 mg/ml solution for injection in pre-filled pen. http://www.medicinesorguk/EMC/medicine/21986/SPC/Victoza+6+mg+ml+solution+for+injection+in+pre-filled+pen. Accessed on 4 May 2015
Food and Drug Administration (2010) Victoza® [liraglutide (rDNA origin) injection], solution for subcutaneous use. https://www.accessdatafdagov/drugsatfda_docs/label/2010/022341lblpdf. Last accessed on 4 May 2015
Rigato M, Fadini GP (2014) Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes 7:107–120. doi:10.2147/DMSO.S37644
Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G (2013) Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 381(9861):117–124. doi:10.1016/S0140-6736(12)61267-7
Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R (2009) Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+ SU): a randomised controlled trial. Diabetologia 52(10):2046–2055. doi:10.1007/s00125-009-1472-y
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L (2009) Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374(9683):39–47. doi:10.1016/S0140-6736(09)60659-0
Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S (2009) Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 26(3):268–278. doi:10.1111/j.1464-5491.2009.02666.x
Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B (2009) Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373(9662):473–481. doi:10.1016/S0140-6736(08)61246-5
Abdul-Ghani MA, Williams K, Kanat M, Altuntas Y, DeFronzo RA (2013) Insulin vs GLP-1 analogues in poorly controlled Type 2 diabetic subjects on oral therapy: a meta-analysis. J Endocrinol Invest 36(3):168–173. doi:10.3275/8367
Barnett AH (2015) NICE draft type 2 diabetes guidelines: a cause for concern. Lancet Diabetes Endocrinol 3:403–405. doi:10.1016/S2213-8587(15)00053-4
National Institute for Health and Care Excellence (NICE) Liraglutide for the treatment of type 2 diabetes mellitus. https://www.niceorguk/guidance/ta203. Accessed on 4 May 2015
Lapolla A, Frison V, Bettio M, Dal Pos M, Rocchini P, Panebianco G, Tadiotto F, Da Tos V, D’Ambrosio M, Marangoni A, Ferrari M, Pianta A, Balzano S, Confortin L, Lamonica M, Marin N, Strazzabosco M, Brun E, Mesturino CA, Simoncini M, Zen F, Bax G, Bonsembiante B, Cardone C, Dal Frà MG, Gallo A, Masin M, Piarulli F, Sartore G, Simioni N (2015) Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clin Ther 37(3):574–584. doi:10.1016/j.clinthera.2014.11.015
Fadini GP, Simioni N, Frison V, Dal Pos M, Bettio M, Rocchini P, Avogaro A (2013) Independent glucose and weight-reducing effects of Liraglutide in a real-world population of type 2 diabetic outpatients. Acta Diabetol 50(6):943–949. doi:10.1007/s00592-013-0489-3
Lee WC, Dekoven M, Bouchard J, Massoudi M, Langer J (2014) Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab 16(9):819–826. doi:10.1111/dom.12285
Ponzani P (2013) Long-term effectiveness and safety of liraglutide in clinical practice. Minerva Endocrinol 38(1):103–112 (R07132039 [pii])
Muscogiuri G, Cignarelli A, Giorgino F, Prodram F, Santi D, Tirabassi G, Balercia G, Modica R, Faggiano A, Colao A (2014) GLP-1: benefits beyond pancreas. J Endocrinol Invest. doi:10.1007/s40618-014-0137-y
Koch L (2013) Diabetes: individualized HbA(1c) targets in elderly patients with T2DM. Nat Rev Endocrinol 9(8):440. doi:10.1038/nrendo.2013.114
Strain WD, Lukashevich V, Kothny W, Hoellinger MJ, Paldanius PM (2013) Individualised treatment targets for elderly patients with type 2 diabetes using vildagliptin add-on or lone therapy (INTERVAL): a 24 week, randomised, double-blind, placebo-controlled study. Lancet 382(9890):409–416. doi:10.1016/S0140-6736(13)60995-2
Morley JE, Sinclair A (2013) Individualising treatment for older people with diabetes. Lancet 382(9890):378–380. doi:10.1016/S0140-6736(13)61055-7
Thong K, Sen Gupta P, Cull M, Adamson K, Dove D, Rowles S, Tarpey S, Duncan C, Chalmers J, Harper R, McDonald P, Brennan U, Walton C, Ryder R, on behalf of the ABCD nationwide exenatide and liraglutide audit contributors (2014) GLP-1 receptor agonists in type 2 diabetes—NICE guidelines versus clinical practice. Br J Diabetes Vasc Dis 14:52–29
Esposito K, Chiodini P, Capuano A, Maiorino MI, Bellastella G, Giugliano D (2014) Baseline glycemic parameters predict the hemoglobin A1c response to DPP-4 inhibitors : meta-regression analysis of 78 randomized controlled trials with 20,053 patients. Endocrine 46(1):43–51. doi:10.1007/s12020-013-0090-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There are no funding received for this study.
Conflict of interest
None.
Ethical approval
The study has been approved by the Ethical Committee of Padova.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Rigato, M., Avogaro, A. & Fadini, G.P. Effects of dose escalating liraglutide from 1.2 to 1.8 mg in clinical practice: a case–control study. J Endocrinol Invest 38, 1357–1363 (2015). https://doi.org/10.1007/s40618-015-0385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0385-5