Abstract
Purpose
Congenital adrenal hyperplasia (CAH) is an autosomal recessive disease characterized by impaired adrenal steroidogenesis and most often caused by CYP21A2 gene mutations. For the first time, we reported complete spectrum and frequency of CYP21A2 gene mutations in 61 unrelated patients with classical and non-classical CAH from Serbia.
Methods
Direct DNA sequencing of whole CYP21A2 gene and polymerase chain reaction with sequence-specific primers for detection of CYP21A1P/CYP21A2 chimeras were combined.
Results
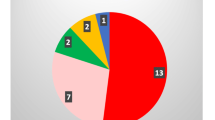
We identified 18 different pathogenic alleles—two of them novel. Mutation detection rate was highest in patients with salt-wasting form of CAH (94.7 %). The most prevalent mutation was intron 2 splice site mutation, c.290-13A/C>G (18.5 %). Other mutation frequencies were: CYP21A1P/CYP21A2 chimeras (13 %), p.P30L (13 %), p.R356W (11.1 %), p.G110fs (7.4 %), p.Q318X (4.6 %), p.V281L (4.6 %), p.I172N (2.8 %), p.L307fs (2.8 %), p.P453S (1.9 %), etc. Mainly, frequencies were similar to those in Slavic populations and bordering countries. However, we found 6.5 % of alleles with multiple mutations, frequently including p.P453S. Effects of novel mutations, c.386T>C (p.Leu129Pro) and c.493T>C (p.Ser165Pro), were characterized in silico as deleterious. The effect of well-known mutations on Serbian patients’ phenotype was as expected.
Conclusions
The first comprehensive molecular genetic study of Serbian CAH patients revealed two novel CYP21A2 mutations. This study will enable genetic counseling in our population and contribute to better understanding of molecular landscape of CAH in Europe.

Similar content being viewed by others
Abbreviations
- 17-OHP:
-
17-Hydroprogesterone
- 21-OH:
-
21-Hydroxylase
- Bp:
-
Base pair
- CAH:
-
Congenital adrenal hyperplasia
- CYP21A2:
-
Cytochrome P450, family 21, subfamily A, polypeptide 2
- dNTP:
-
Deoxyribonucleoside triphosphate
- MLPA:
-
Multiplex ligation-dependent probe amplification
- HGMD:
-
Human gene mutation database
- NC-CAH:
-
Non-classical form of CAH
- PCR:
-
Polymerase chain reaction
- PCR-SSP:
-
Sequence-specific PCR amplification
- SV-CAH:
-
Simple virilizing form of CAH
- SW-CAH:
-
Salt-wasting form of CAH
- UTR:
-
Untranslated region
References
Concolino P, Mello E, Zuppi C, Capoluongo E (2010) Molecular diagnosis of congenital adrenal hyperplasia due to 21-hydroxylase deficiency: an update of new CYP21A2 mutations. Clin Chem Lab Med 48:1057–1062. doi:10.1515/cclm.2010.239
Gonçalves J, Friães A, Moura L (2007) Congenital adrenal hyperplasia: focus on the molecular basis of 21-hydroxylase deficiency. Expert Rev Mol Med 9:1–23. doi:10.1017/s1462399407000300
Rabbani B, Mahdieh N, Haghi Ashtiani MT, Akbari MT, Rabbani A (2011) Molecular diagnosis of congenital adrenal hyperplasia in Iran: focusing on CYP21A2 gene. Iran J Pediatr 21:139–150
Trapp CM, Oberfield SE (2012) Recommendations for treatment of nonclassic congenital adrenal hyperplasia (NCCAH): an update. Steroids 77:342–346. doi:10.1016/j.steroids.2011.12.009
Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY (1999) Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J Biol Chem 274:12147–12156
New MI, Lekarev O, Mancenido D, Parsa A, Yuen T (2013) Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. In: New MI, Lekarev O, Parsa A, O’Malley B, Hammer GD (eds) Genetic steroid disorders. Elsevier, New York, pp 29–51
Tusié-Luna MT, White PC (1995) Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci USA 92:10796–10800
Krone N, Arlt W (2009) Genetics of congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab 23:181–192. doi:10.1016/j.beem.2008.10.014
Sharaf S, Hafez M, ElAbd D, Ismail A, Thabet G, Elsharkawy M (2014) High frequency of splice site mutation in 21-hydroxylase deficiency children. J Endocrinol Invest. doi:10.1007/s40618-014-0207-1
Krone N, Braun A, Weinert S, Peter M, Roscher AA, Partsch CJ, Sippell WG (2002) Multiplex minisequencing of the 21-hydroxylase gene as a rapid strategy to confirm congenital adrenal hyperplasia. Clin Chem 48:818–825
Dolzan V, Stopar-Obreza M, Zerjav-Tansek M, Breskvar K, Krzisnik C, Battelino T (2003) Mutational spectrum of congenital adrenal hyperplasia in Slovenian patients: a novel Ala15Thr mutation and Pro30Leu within a larger gene conversion associated with a severe form of the disease. Eur J Endocrinol 149:137–144
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi:10.1038/msb.2011.75
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. doi:10.1093/bioinformatics/bti770
Robins T, Carlsson J, Sunnerhagen M, Wedell A, Persson B (2006) Molecular model of human CYP21 based on mammalian CYP2C5: structural features correlate with clinical severity of mutations causing congenital adrenal hyperplasia. Mol Endocrinol 20:2946–2964. doi:10.1210/me.2006-0172
Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, Lesser M, New MI, White PC (1992) Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest 90:584–595. doi:10.1172/jci115897
Wedell A, Thilén A, Ritzén EM, Stengler B, Luthman H (1994) Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 78:1145–1152. doi:10.1210/jcem.78.5.8175971
Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP (2000) Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab 85:1059–1065. doi:10.1210/jcem.85.3.6441
Cavarzere P, Vincenzi M, Teofoli F, Gaudino R, Lauriola S, Maines E, Camilot M, Antoniazzi F (2013) Genotype in the diagnosis of 21-hydroxylase deficiency: who should undergo CYP21A2 analysis? J Endocrinol Invest 36:1083–1089. doi:10.3275/9096
Balraj P, Lim PG, Sidek H, Wu LL, Khoo AS (2013) Mutational characterization of congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Malaysia. J Endocrinol Invest 36:366–374. doi:10.3275/8648
Wilson RC, Mercado AB, Cheng KC, New MI (1995) Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. J Clin Endocrinol Metab 80:2322–2329. doi:10.1210/jcem.80.8.7629224
Pérez B, Rodríguez-Pascau L, Vilageliu L, Grinberg D, Ugarte M, Desviat LR (2010) Present and future of antisense therapy for splicing modulation in inherited metabolic disease. J Inherit Metab Dis 33:397–403. doi:10.1007/s10545-010-9135-1
Wilson RC, Nimkarn S, Dumic M, Obeid J, Azar MR, Azar M, Najmabadi H, Saffari F, New MI (2007) Ethnic-specific distribution of mutations in 716 patients with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Mol Genet Metab 90:414–421. doi:10.1016/j.ymgme.2006.12.005
Anastasovska V, Kocova M (2010) Genotype-phenotype correlation in CAH patients with severe CYP21A2 point mutations in the Republic of Macedonia. J Pediatr Endocrinol Metab 23:921–926
Dolzan V, Sólyom J, Fekete G, Kovács J, Rakosnikova V, Votava F, Lebl J, Pribilincova Z, Baumgartner-Parzer SM, Riedl S, Waldhauser F, Frisch H, Stopar-Obreza M, Krzisnik C, Battelino T (2005) Mutational spectrum of steroid 21-hydroxylase and the genotype-phenotype association in Middle European patients with congenital adrenal hyperplasia. Eur J Endocrinol 153:99–106. doi:10.1530/eje.1.01944
Grigorescu Sido A, Weber MM, Grigorescu Sido P, Clausmeyer S, Heinrich U, Schulze E (2005) 21-Hydroxylase and 11beta-hydroxylase mutations in Romanian patients with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab 90:5769–5773. doi:10.1210/jc.2005-0379
Baş F, Kayserili H, Darendeliler F, Uyguner O, Günöz H, Yüksel Apak M, Atalar F, Bundak R, Wilson RC, New MI, Wollnik B, Saka N (2009) CYP21A2 gene mutations in congenital adrenal hyperplasia: genotype-phenotype correlation in Turkish children. J Clin Res Pediatr Endocrinol 1:116–128. doi:10.4008/jcrpe.v1i3.49
Dracopoulou-Vabouli M, Maniati-Christidi M, Dacou-Voutetakis C (2001) The spectrum of molecular defects of the CYP21 gene in the Hellenic population: variable concordance between genotype and phenotype in the different forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:2845–2848. doi:10.1210/jcem.86.6.7574
Barbat B, Bogyo A, Raux-Demay MC, Kuttenn F, Boué J, Simon-Bouy B, Serre JL, Mornet E (1995) Screening of CYP21 gene mutations in 129 French patients affected by steroid 21-hydroxylase deficiency. Hum Mutat 5:126–130. doi:10.1002/humu.1380050205
Carrera P, Bordone L, Azzani T, Brunelli V, Garancini MP, Chiumello G, Ferrari M (1996) Point mutations in Italian patients with classic, non-classic, and cryptic forms of steroid 21-hydroxylase deficiency. Hum Genet 98:662–665
Ezquieta B, Oliver A, Gracia R, Gancedo PG (1995) Analysis of steroid 21-hydroxylase gene mutations in the Spanish population. Hum Genet 96:198–204
Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP (2011) Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 96:E161–E172. doi:10.1210/jc.2010-0319
Dain LB, Buzzalino ND, Oneto A, Belli S, Stivel M, Pasqualini T, Minutolo C, Charreau EH, Alba LG (2002) Classical and nonclassical 21-hydroxylase deficiency: a molecular study of Argentine patients. Clin Endocrinol (Oxf) 56:239–245
Haider S, Islam B, D’Atri V, Sgobba M, Poojari C, Sun L, Yuen T, Zaidi M, New MI (2013) Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci USA 110:2605–2610. doi:10.1073/pnas.1221133110
New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, Sun L, Zaidi M, Wilson RC, Yuen T (2013) Genotype-phenotype correlation in 1507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci USA 110:2611–2616. doi:10.1073/pnas.1300057110
White PC, Speiser PW (2000) Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev 21:245–291. doi:10.1210/edrv.21.3.0398
Kleinle S, Lang R, Fischer GF, Vierhapper H, Waldhauser F, Födinger M, Baumgartner-Parzer SM (2009) Duplications of the functional CYP21A2 gene are primarily restricted to Q318X alleles: evidence for a founder effect. J Clin Endocrinol Metab 94:3954–3958. doi:10.1210/jc.2009-0487
Lekarev O, Tafuri K, Lane AH, Zhu G, Nakamoto JM, Buller-Burckle AM, Wilson TA, New MI (2013) Erroneous prenatal diagnosis of congenital adrenal hyperplasia owing to a duplication of the CYP21A2 gene. J Perinatol 33:76–78. doi:10.1038/jp.2012.5
Dipple KM, McCabe ER (2000) Phenotypes of patients with “simple” Mendelian disorders are complex traits: thresholds, modifiers, and systems dynamics. Am J Hum Genet 66:1729–1735. doi:10.1086/302938
Scriver CR, Waters PJ (1999) Monogenic traits are not simple: lessons from phenylketonuria. Trends Genet 15:267–272
Chen W, Xu Z, Sullivan A, Finkielstain GP, Van Ryzin C, Merke DP, McDonnell NB (2012) Junction site analysis of chimeric CYP21A1P/CYP21A2 genes in 21-hydroxylase deficiency. Clin Chem 58:421–430. doi:10.1373/clinchem.2011.174037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia (Grant No. III41004).
Ethical approval
This study involved human participants and has been approved by the Ethics Committee of the School of Medicine, University of Belgrade in Belgrade, Serbia and the Ethics Committee of the Mother and Child Health Care Institute of Serbia “Dr Vukan Cupic” in Belgrade, Serbia, and has therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Milacic, I., Barac, M., Milenkovic, T. et al. Molecular genetic study of congenital adrenal hyperplasia in Serbia: novel p.Leu129Pro and p.Ser165Pro CYP21A2 gene mutations. J Endocrinol Invest 38, 1199–1210 (2015). https://doi.org/10.1007/s40618-015-0366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0366-8



