Abstract
Purpose
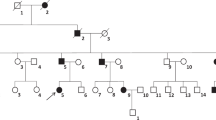
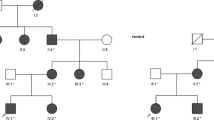
Familial neurohypophyseal diabetes insipidus (FNDI) is a rare, autosomal dominant, inherited disorder which is characterized by severe polydipsia and polyuria generally presenting in early childhood. In the present study, we aimed to analyze the AVP gene in a Turkish family with FNDI.
Methods
Four patients with neurohypophyseal diabetes insipidus and ten healthy members of the family were studied. Diabetes insipidus was diagnosed by the water deprivation test in affected family members. Mutation analysis was performed by sequencing the whole coding region of AVP-NPII gene using DNA isolated from peripheral blood samples.
Results
Urine osmolality was low (<300 mOsm/kg) during water deprivation test, and an increase more than 50 % in urine osmolality and recovery of the symptoms were observed by the administration of desmopressin in all patients. Plasma copeptin levels were lower than expected according to plasma osmolality. Pituitary MRI revealed partial empty sella with a bright spot in index patient and a normal neurohypophysis in the other affected subjects. Genetic screening revealed a novel, heterozygous mutation designated as c.-3A>C in all patients.
Conclusion
c.-3A>C mutation in 5′UTR of AVP gene in this family might lead to the truncation of signal peptide, aggregation of AVP in the cytoplasm instead of targeting in the endoplasmic reticulum, thereby could disrupt AVP secretion without causing neuronal cytotoxicity, which might explain the presence of bright spot. The predicted effect of this mutation should be investigated by further in vitro molecular studies.




Similar content being viewed by others
References
Christensen JH, Siggaard C, Corydon TJ, Robertson GL, Gregersen N, Bolund L, Rittig S (2004) Differential cellular handling of defective arginine vasopressin (AVP) prohormones in cells expressing mutations of the AVP gene associated with autosomal dominant and recessive familial neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab 89(9):4521–4531. doi:10.1210/jc.2003-031813
Mohr E, Hillers M, Ivell R, Haulica ID, Richter D (1985) Expression of the vasopressin and oxytocin genes in human hypothalami. FEBS Lett 193(1):12–16
Land H, Schutz G, Schmale H, Richter D (1982) Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin–neurophysin II precursor. Nature 295(5847):299–303
Riddell DC, Mallonee R, Phillips JA, Parks JS, Sexton LA, Hamerton JL (1985) Chromosomal assignment of human sequences encoding arginine vasopressin–neurophysin II and growth hormone releasing factor. Somat Cell Mol Genet 11(2):189–195
Schmale H, Ivell R, Breindl M, Darmer D, Richter D (1984) The mutant vasopressin gene from diabetes insipidus (Brattleboro) rats is transcribed but the message is not efficiently translated. EMBO J 3(13):3289–3293
Acher R, Chauvet J, Rouille Y (2002) Dynamic processing of neuropeptides: sequential conformation shaping of neurohypophysial preprohormones during intraneuronal secretory transport. J Mol Neurosci MN 18(3):223–228. doi:10.1385/JMN:18:3:223
Ito M, Mori Y, Oiso Y, Saito H (1991) A single base substitution in the coding region for neurophysin II associated with familial central diabetes insipidus. J Clin Investig 87(2):725–728. doi:10.1172/JCI115052
Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN (2014) The human gene mutation database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133(1):1–9. doi:10.1007/s00439-013-1358-4
Fenske W, Allolio B (2012) Clinical review: current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab 97(10):3426–3437. doi:10.1210/jc.2012-1981
Rittig S, Robertson GL, Siggaard C, Kovacs L, Gregersen N, Nyborg J, Pedersen EB (1996) Identification of 13 new mutations in the vasopressin–neurophysin II gene in 17 kindreds with familial autosomal dominant neurohypophyseal diabetes insipidus. Am J Hum Genet 58(1):107–117
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Pedersen AG, Nielsen H (1997) Neural network prediction of translation initiation sites in eukaryotes: perspectives for EST and genome analysis. Proc Int Conf Intell Syst Mol Biol 5:226–233
Czaczkes JW, Kleeman CR, Koenig M (1964) Physiologic studies of antidiuretic hormone by its direct measurement in human plasma. J Clin Investig 43:1625–1640. doi:10.1172/JCI105038
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52(1):112–119. doi:10.1373/clinchem.2005.060038
Robertson GL, Mahr EA, Athar S, Sinha T (1973) Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Investig 52(9):2340–2352. doi:10.1172/JCI107423
Fenske W, Quinkler M, Lorenz D, Zopf K, Haagen U, Papassotiriou J, Pfeiffer AF, Fassnacht M, Stork S, Allolio B (2011) Copeptin in the differential diagnosis of the polydipsia-polyuria syndrome—revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab 96(5):1506–1515. doi:10.1210/jc.2010-2345
Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J (2011) Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 96(4):1046–1052. doi:10.1210/jc.2010-2499
Morgenthaler NG, Struck J, Jochberger S, Dunser MW (2008) Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab TEM 19(2):43–49. doi:10.1016/j.tem.2007.11.001
Imura H, Nakao K, Shimatsu A, Ogawa Y, Sando T, Fujisawa I, Yamabe H (1993) Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med 329(10):683–689. doi:10.1056/NEJM199309023291002
Cacciari E, Zucchini S, Carla G, Pirazzoli P, Cicognani A, Mandini M, Busacca M, Trevisan C (1990) Endocrine function and morphological findings in patients with disorders of the hypothalamo-pituitary area: a study with magnetic resonance. Arch Dis Child 65(11):1199–1202
Gudinchet F, Brunelle F, Barth MO, Taviere V, Brauner R, Rappaport R, Lallemand D (1989) MR imaging of the posterior hypophysis in children. AJR Am J Roentgenol 153(2):351–354. doi:10.2214/ajr.153.2.351
Moses AM, Clayton B, Hochhauser L (1992) Use of T1-weighted MR imaging to differentiate between primary polydipsia and central diabetes insipidus. AJNR Am J Neuroradiol 13(5):1273–1277
Hannon MJ, Orr C, Moran C, Behan LA, Agha A, Ball SG, Thompson CJ (2012) Anterior hypopituitarism is rare and autoimmune disease is common in adults with idiopathic central diabetes insipidus. Clin Endocrinol 76(5):725–728. doi:10.1111/j.1365-2265.2011.04270.x
Lee YW, Lee KW, Ryu JW, Mok JO, Ki CS, Park HK, Kim YJ, Kim SJ, Byun DW, Suh KI, Yoo MH, Shin HB, Lee YK, Kim CH (2008) Mutation of Glu78 of the AVP-NPII gene impairs neurophysin as a carrier protein for arginine vasopressin in a family with neurohypophyseal diabetes insipidus. Ann Clin Lab Sci 38(1):12–14
Birkegaard C, Christensen JH, Falorni A, Marzotti S, Minarelli V, Gregersen N, Rittig S (2013) A novel variation in the AVP gene resulting in familial neurohypophyseal diabetes insipidus in a large Italian kindred. Pituitary 16(2):152–157. doi:10.1007/s11102-012-0392-x
De Angioletti M, Lacerra G, Sabato V, Carestia C (2004) Beta+ 45 G → C: a novel silent beta-thalassaemia mutation, the first in the Kozak sequence. Br J Haematol 124(2):224–231
Kozak M (1986) Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44(2):283–292
Christensen JH, Siggaard C, Corydon TJ, Robertson GL, Gregersen N, Bolund L, Rittig S (2004) Impaired trafficking of mutated AVP prohormone in cells expressing rare disease genes causing autosomal dominant familial neurohypophyseal diabetes insipidus. Clin Endocrinol 60(1):125–136
Birk J, Friberg MA, Prescianotto-Baschong C, Spiess M, Rutishauser J (2009) Dominant pro-vasopressin mutants that cause diabetes insipidus form disulfide-linked fibrillar aggregates in the endoplasmic reticulum. J Cell Sci 122(Pt 21):3994–4002. doi:10.1242/jcs.051136
Russell TA, Ito M, Ito M, Yu RN, Martinson FA, Weiss J, Jameson JL (2003) A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Investig 112(11):1697–1706. doi:10.1172/JCI18616
Yan Z, Hoffmann A, Kaiser EK, Grunwald WC Jr, Cool DR (2011) Misfolding of mutated vasopressin causes ER-retention and activation of ER-stress markers in Neuro-2a cells. Open Neuroendocrinol J 4:136–146. doi:10.2174/1876528901104010136
Fujisawa I (2004) Magnetic resonance imaging of the hypothalamic-neurohypophyseal system. J Neuroendocrinol 16(4):297–302. doi:10.1111/j.0953-8194.2004.01183.x
Hayashi M, Arima H, Ozaki N, Morishita Y, Hiroi M, Ozaki N, Nagasaki H, Kinoshita N, Ueda M, Shiota A, Oiso Y (2009) Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 296(5):R1641–R1649. doi:10.1152/ajpregu.00034.2009
de Fost M, van Trotsenburg AS, van Santen HM, Endert E, van den Elzen C, Kamsteeg EJ, Swaab DF, Fliers E (2011) Familial neurohypophyseal diabetes insipidus due to a novel mutation in the arginine vasopressin–neurophysin II gene. Eur J Endocrinol 165(1):161–165. doi:10.1530/EJE-11-0048
DiMeglio LA, Gagliardi PC, Browning JE, Quigley CA, Repaske DR (2001) A missense mutation encoding cys(67) – > gly in neurophysin ii is associated with early onset autosomal dominant neurohypophyseal diabetes insipidus. Mol Genet Metab 72(1):39–44. doi:10.1006/mgme.2000.3117
Christensen JH, Siggaard C, Corydon TJ, deSanctis L, Kovacs L, Robertson GL, Gregersen N, Rittig S (2004) Six novel mutations in the arginine vasopressin gene in 15 kindreds with autosomal dominant familial neurohypophyseal diabetes insipidus give further insight into the pathogenesis. Eur J Hum Genet: EJHG 12(1):44–51. doi:10.1038/sj.ejhg.5201086
Acknowledgments
The manuscript was edited for proper English language by a highly qualified native English speaking editor in Bezmialem Vakif University Professional English for Medical Purpose (BVUPSO-101-03/15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was conducted in compliance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ilhan, M., Tiryakioglu, N.O., Karaman, O. et al. A novel AVP gene mutation in a Turkish family with neurohypophyseal diabetes insipidus. J Endocrinol Invest 39, 285–290 (2016). https://doi.org/10.1007/s40618-015-0357-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0357-9