Abstract
Context
Safety of intravenous (IV) steroid pulses in patients with Graves’ orbitopathy (GO) is still controversial while steroid dose and treatment application have not been finalized. Frequency, severity and characterization of adverse events (AE) were prospectively analyzed.
Setting
Academic referral orbital center with a joint thyroid-eye clinic.
Patients
Eighty consecutive and unselected patients with active and severe GO.
Methods
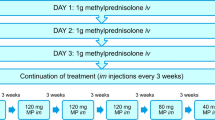
During an established treatment with IV methylprednisolone (cumulative dose 4.5 g) occurring AE were prospectively coded according to the standardized and recognized medical dictionary for regulatory activities (MedDRA). Outcome and severity of AE were documented. AEs judged as at least possibly related to drug treatment were graded as side effect (SE). AEs matching a seriousness criteria as defined by the ICH guideline E6 (good clinical practice) were graded as serious.
Results
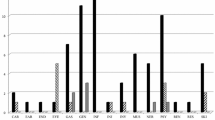
A total of 38.75 % (31/80) of the treated GO patients reported at least one AE while 18 patients (22.5 %) reported at least one SE. All SE were within the safety profile of IV methylprednisolone; 31/32 SE (96.87 %) were mild-moderate and reversible and only 1/80 patient (1.25 %) stopped steroid treatment due to exacerbation of her depression. Most AE were accessory symptoms of the underlying disease and a few only were directly related to IV steroids. Most AEs (90.6 %) were graded as mild. Only six patients (7.5 %) were hospitalized, three of them due to a dysthyroid optic neuropathy.
Conclusions
Prospective and standardized evaluation with MedDRA and the ICH guideline demonstrated the good pharmacological tolerance and low morbidity of this moderate steroid regimen.


Similar content being viewed by others
References
Bartalena L, Fatourechi V (2014) Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest 37:691–700
Piantanida E, Tanda ML, Lai A, Sassi L, Bartalena L (2013) Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest 36:444–449
Bartalena L, Baldeschi L, Dickinson AJ, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits MP, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas G, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM (2008) Consensus statement of the European group on Graves’ orbitopathy (EUGOGO) on management of Graves’ orbitopathy. Thyroid 18:333–346
Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, Curro N, Daumerie C, Kahaly GJ, Krassas GE, Lane CM, Lazarus JH, Marino M, Nardi M, Neoh C, Orgiazzi J, Pearce S, Pinchera A, Pitz S, Salvi M, Sivelli P, Stahl M, von Arx G, Wiersinga WM, European Group on Graves O (2008) Consensus statement of the European Group on Graves’ orbitopathy (EUGOGO) on management of GO. Eur J Endocrinol 158:273–285
Methylprednisolone 1000 mg powder and solvent for solution for injection/infusion (2014) Summary of product characteristic
Melamud B, Lurie Y, Goldin E, Levi I, Esayag Y (2014) Methylprednisolone-induced liver injury: a diagnostic challenge. Isr Med Assoc J 16:180–181
Marino M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14:403–406
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10:521
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S, Beck-Peccoz P (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14:631–634
Marino M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, Campani D, Vitti P, Bartalena L, Pincheral A, Marcocci C (2005) Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves’ ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest 28:280–284
Owecki M, Sowinski J (2006) Acute myocardial infarction during high-dose methylprednisolone therapy for Graves’ ophthalmopathy. Pharm World Sci 28:73–75
Gursoy A, Cesur M, Erdogan MF, Corapcioglu D, Kamel N (2006) New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with Graves’ ophthalmopathy. Endocrine 29:513–516
Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G (2001) High-dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 24:152–158
Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, Barbesino G, Mazzi B, Bartolomei MP, Lepri P, Cartei F, Nardi M, Pinchera A (2001) Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 86:3562–3567
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G, Azzolini C, Boboridis KG, Mourits MP, Soeters MR, Baldeschi L, Nardi M, Curro N, Boschi A, Bernard M, von Arx G, European Group on Graves O (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97:4454–4463
van Geest RJ, Sasim IV, Koppeschaar HP, Kalmann R, Stravers SN, Bijlsma WR, Mourits MP (2008) Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol 158:229–237
Kahaly GJ, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240
Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L (2009) Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab 94:2708–2716
Zang S, Ponto KA, Pitz S, Kahaly GJ (2011) Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 34:876–880
Zang S, Ponto KA, Kahaly GJ (2011) Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96:320–332
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, Bartalena L, European Group of Graves O (2012) Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol 166:247–253
ICH (2001) Data elements for transmission of individual case safety reports E2B. In: International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use
MSSO (2013) MedDRA Term selection: points to consider Release 4.6 Based on MedDRA Version 16.1
ICH (1996) Guideline for good clinical practice E6(R1). In: International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use
Menconi F, Marino M, Pinchera A, Rocchi R, Mazzi B, Nardi M, Bartalena L, Marcocci C (2007) Effects of total thyroid ablation versus near-total thyroidectomy alone on mild to moderate Graves’ orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab 92:1653–1658
Wichary H, Gasinska T (2012) Methylprednisolone and hepatotoxicity in Graves’ ophthalmopathy. Thyroid 22:64–69
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17:357–362
Lendorf ME, Rasmussen AK, Fledelius HC, Feldt-Rasmussen U (2009) Cardiovascular and cerebrovascular events in temporal relationship to intravenous glucocorticoid pulse therapy in patients with severe endocrine ophthalmopathy. Thyroid 19:1431–1432
Conflict of interest
The authors have nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riedl, M., Kolbe, E., Kampmann, E. et al. Prospectively recorded and MedDRA-coded safety data of intravenous methylprednisolone therapy in Graves’ orbitopathy. J Endocrinol Invest 38, 177–182 (2015). https://doi.org/10.1007/s40618-014-0227-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-014-0227-x