Abstract
Purpose
Intravenous glucocorticoids (GCs) are the mainstay of treatment for severe forms of Graves’ orbitopathy (GO). Our aim was to assess the effectiveness and safety of a modified monthly regimen (mMR) and to compare them with those of the established weekly regimen (WR).
Methods
This was a prospective non-randomized single-center study involving 62 patients, divided into two therapeutic groups depending on their referral time. Thirty-one subjects, admitted in the period 2017–2018, were treated with mMR, total dose—5.5 g, with intake of oral GCs after completion of intravenous infusions. Thirty subjects, who were referred in the period 2019–2020, were treated with WR, total dose—4.5 g One patient refused to be part of the WR group and was treated with mMR. Eye status and therapeutic response were evaluated on the 1st, 3rd and 6th months, quality of life—at 3rd and 6th month.
Results
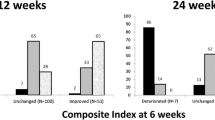
At 1st month and 3rd month, there was no significant difference in the therapeutic response between the two groups. At 3rd month, the proportion of patients with improvement in soft tissue manifestations and subjective complaints was significantly higher in mMR group (65.6% vs. 40% and 81.3% vs. 46.7%, respectively) and the same manifestations were of significantly milder degree.
At 3rd month, significant improvement in quality of life was found without significant difference between the two groups.
At 6th month, worsening of GO occurred in 3 patients from WR group, while in 5 patients from mMR group further improvement was found.
Conclusions
The two GC regimens have comparable efficacy with small differences in the time of onset of the effect and its duration, as well as in the effectiveness on some ocular manifestations.
Trial registration number NCT05793359/29.03.2023, retrospectively registered..
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, M.S., upon reasonable request.
References
van Geest RJ, Sasim IV, Koppeschaar HPF, Kalmann R, Stravers SN, Bijlsma WR, Mourits MP (2008) Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo-controlled study. Eur J Endocrinol 158:229–237. https://doi.org/10.1530/EJE-07-0558
Macchia PE, Bagattini M, Lupoli G, Vitale M, Vitale G, Fenzi G (2001) High-dose intravenous corticosteroid therapy for Graves’ ophthalmopathy. J Endocrinol Invest 24:152–158. https://doi.org/10.1007/BF03343835
Kahaly GJ, Pitz S, Hommel G, Dittmar M (2005) Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab 90:5234–5240. https://doi.org/10.1210/jc.2005-0148
Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell’Unto E, Rocchi R, Barbesino G, Mazzi B, Bartolomei MP, Lepri P (2001) Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves ’ ophthalmopathy : results of a prospective, single-blind, randomized study. J Clin Endocrinol Metab 86:3562–3567. https://doi.org/10.1210/jcem.86.8.7737
Aktaran Ş, Akarsu E, Erbağci İ, Araz M, Okumuş S, Kartal M (2006) Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract 61:45–51. https://doi.org/10.1111/j.1742-1241.2006.01004.x
Akarsu E, Buyukhatipoglu H, Aktaran Ş, Kurtul N (2011) Effects of pulse methylprednisolone and oral methylprednisolone treatments on serum levels of oxidative stress markers in Graves’ ophthalmopathy. Clin Endocrinol 74:118–124. https://doi.org/10.1111/j.1365-2265.2010.03904.x
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, Marinò M, Vaidya B, Wiersinga WM (2021) The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol 185:G43-67. https://doi.org/10.1530/EJE-21-0479
Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, Perros P, Salvi M, Wiersinga WM (2016) The 2016 European thyroid association/European group on Graves’ orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 55:9–269. https://doi.org/10.1159/000443828
Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, Bournaud C, Stahl M, Sassi L, Veronesi G et al (2012) Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 97:4454–4463. https://doi.org/10.1210/jc.2012-2389
Nedeljkovic Beleslin B, Ciric J, Stojkovic M, Savic S, Lalic T, Stojanovic M, Miletic M, Knezevic M, Stankovic B (2020) Comparison of efficacy and safety of parenteral versus parenteral and oral glucocorticoid therapy in Graves’ orbitopathy. Int J Clin Pract 74:0–2. https://doi.org/10.1111/ijcp.13608
Sánchez-Ortiga R, Moreno-Pérez Ó, González Sánchez V, Arias Mendoza N, Mauri Dot M, Alfayate Guerra R, López Macia A, Picó Alfonso A (2009) Treatment of Graves’ ophthalmopathy with high-dose intravenous methylprednisolone: a comparison of two dosing regimens. Endocrinol Y Nutr 56(3):118–122. https://doi.org/10.1016/S1575-0922(09)70841-1
Zhu W, Ye L, Shen L, Jiao Q, Huang F, Han R, Zhang X, Wang S, Wang W, Ning G (2014) A prospective, randomized trial of intravenous glucocorticoids therapy with different protocols for patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab 99:1999–2007. https://doi.org/10.1210/jc.2013-3919
Mu P-W, Tang X-X, Wang Y-N, Lin S, Wang MM, Yin QL, Shu J, Zhu BL, Li JR, Zhou L (2020) Comparison of two regimens for patients with thyroid-associated ophthalmopathy receiving intravenous methyl prednisolone: a single center prospective randomized trial. Exp Ther Med 20:1–9. https://doi.org/10.3892/etm.2020.9282
Roy A, Dutta D, Ghosh S, Mukhopadhyay P, Mukhopadhyay S, Chowdhury S (2015) Efficacy and safety of low dose oral prednisolone as compared to pulse intravenous methylprednisolone in managing moderate severe Graves’ orbitopathy: a randomized controlled trial. Indian J Endocrinol Metab 19:351–358. https://doi.org/10.4103/2230-8210.152770
Jia J, Dong J, Deng L (2022) Network meta-analysis of different intravenous glucocorticoid regimes for the treatment of Graves’ orbitopathy. Front Pharmacol 13:1–9. https://doi.org/10.3389/fphar.2022.785757
Lendorf ME, Rasmussen ÅK, Fledelius HC, Feldt-Rasmussen U (2009) Cardiovascular and cerebrovascular events in temporal relationship to intravenous glucocorticoid pulse therapy in patients with severe endocrine ophthalmopathy. Thyroid 19:1431–1432. https://doi.org/10.1089/thy.2009.0069
Weissel M, Hauff W (2000) Fatal liver failure after high-dose glucocorticoid pulse therapy in a patient with severe thyroid eye disease. Thyroid 10:521. https://doi.org/10.1089/thy.2000.10.521
Marinól M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marocci C (2004) Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophthalmopathy. Thyroid 14:403–406. https://doi.org/10.1089/105072504774193276
Salvi M, Vannucchi G, Sbrozzi F, Del Castello AB, Carnevali A, Fargion S, Beck-Peccoz P (2004) Onset of autoimmune hepatitis during intravenous steroid therapy for thyroid-associated ophthalmopathy in a patient with Hashimoto’s thyroiditis: case report. Thyroid 14:631–634. https://doi.org/10.1089/1050725041692927
Marinò M, Morabito E, Altea MA, Ambrogini E, Oliveri F, Brunetto MR, Pollina LE, Campani D, Vitti P, Bartalena L (2005) Autoimmune hepatitis during intravenous glucocorticoid pulse therapy for Graves’ ophthalmopathy treated successfully with glucocorticoids themselves. J Endocrinol Invest 28:280–284. https://doi.org/10.1007/BF03345386
Le Moli R, Baldeschi L, Saeed P, Regensburg N, Mourits MP, Wiersinga WM (2007) Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves’ ophthalmopathy. Thyroid 17:357–362. https://doi.org/10.1089/thy.2006.0267
Gursoy A, Cesur M, Erdogan MF, Çorapcioglu D, Kamel N (2006) New-onset acute heart failure after intravenous glucocorticoid pulse therapy in a patient with Graves’ ophthalmopathy. Endocrine 29:513–516. https://doi.org/10.1385/ENDO:29:3:513
Owecki M, Sowiński J (2006) Acute myocardial infarction during high-dose methylprednisolone therapy for Graves’ ophthalmopathy. Pharm World Sci 28:73–75. https://doi.org/10.1007/s11096-006-9013-y
Zang S, Ponto KA, Kahaly GJ (2011) Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab 96:320–332. https://doi.org/10.1210/jc.2010-1962
He Y, Mu K, Liu R, Zhang J, Xiang N (2017) Comparison of two different regimens of intravenous methylprednisolone for patients with moderate to severe and active Graves’ ophthalmopathy: a prospective, randomized controlled trial. Endocr J 64:141–149. https://doi.org/10.1507/endocrj.EJ16-0083
Bartalena L, Veronesi G, Krassas GE, Wiersinga WM, Marcocci C, Marinò M, Salvi M, Daumerie C, Bournaud C, Stahl M et al (2017) Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate-to-severe and active Graves’ orbitopathy? J Endocrinol Invest 40:547–553. https://doi.org/10.1007/s40618-017-0608-z
Zangi S, Ponto KA, Pitz S, Kahaly GJ (2011) Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest 34:876–880. https://doi.org/10.1007/BF03346732
Hoppe E, Lee ACH, Hoppe D, Kahaly GJ (2021) Predictive factors for changes in quality of life after steroid treatment for active moderate-to-severe Graves’ orbitopathy: a prospective trial. Eur Thyroid J 9:313–320. https://doi.org/10.1159/000508071
Tsirouki T, Bargiota A, Tigas S, Vasileiou A, Kapsalaki E, Giotaki Z, Asproudis I, Tsatsoulis A, Koukoulis G, Tsironi EE (2016) Clinical and imaging evaluation of the response to intravenous steroids in patients with Graves’ orbitopathy and analysis on who requires additional therapy. Clin Opthalmol 10:2277–2289. https://doi.org/10.2147/OPTH.S118555
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, Material preparation was performed by MAS, IDD, IAY whereas data collection—by all authors. The first draft of the manuscript was written by MAS and all authors commented on previous versions of the manuscript. All authors read approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study protocol was approved by the Institutional Ethical Committee (approval number 21A/25.07.2017) and was in accordance with the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All patients included in the study signed an informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stoynova, M.A., Shinkov, A.D., Dimitrova, I.D. et al. Comparison of the efficacy of two different glucocorticoid regimens for treatment of active moderate-to-severe Graves’ orbitopathy. Int Ophthalmol 43, 4747–4757 (2023). https://doi.org/10.1007/s10792-023-02875-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02875-z