Abstract
Objective
Data on treatment outcomes among minority populations treated with remdesivir are limited. We sought to evaluate outcomes among patients hospitalized with COVID-19 and treated with remdesivir among a predominantly Black and LatinX population.
Methods
This was a retrospective cohort study of adult patients hospitalized with COVID-19 and treated with remdesivir at an urban hospital in Newark, NJ, between May 1, 2020, and April 30, 2021, prior to widespread COVID-19 vaccination uptake. We describe 28-day mortality by demographic, socio-economic, and clinical factors, including clinical status by World Health Organization’s (WHO) 8-point Ordinal Scale for Clinical Improvement.
Results
A total of 206 patients met study inclusion criteria (52% were male, 41% non-Hispanic Black and 42% Hispanic). Overall mortality at 28 days was 11%. Eighty-one percent of patients with baseline WHO status of 4 or greater recovered by day 14. Mortality was higher among those who were older (p = 0.01), those with underlying diabetes mellitus (p = 0.047), those with more severe illness on admission by WHO Ordinal Scale (WHO status ≥ 4), and those on concomitant tociluzimab or convalescent plasma use.
Conclusions
We found that remdesivir was effective in treating most COVID-19 patients in our study. Traditional risk factors, such as advanced age and underlying co-morbidities, were associated with worse clinical outcomes and deaths.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical outcomes among patients hospitalized for COVID-19 have been influenced by various factors, including underlying co-morbidities, age, sex, socio-economic factors, laboratory abnormalities, treatment, pathogenicity of the virus strain, and COVID-19 vaccination status [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The use of SARS-CoV-2 antivirals, such as remdesivir (RDV), has been shown to provide clinical benefit in shortening the time to clinical recovery among patients hospitalized with COVID-19 [15,16,17]. RDV works by inhibiting the viral RNA-dependent, RNA polymerase, thereby preventing the virus from replication [18]. It was granted an Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA) in May 2020 for the treatment of COVID-19 in hospitalized patients [19]. Currently, COVID-19 Treatment Guidelines by the National Institutes of Health [20] recommends the use of RDV in hospitalized adults with COVID-19 who require oxygen supplementation or who are at high risk of progressing to severe COVID-19. The use of RDV in combination with other treatments, such as dexamethasone, has also been shown to improve outcomes in hospitalized patients with COVID-19 [20].
The COVID-19 pandemic has disproportionately impacted certain racial, ethnic, and socioeconomic groups, and has highlighted disparities in healthcare, housing, and other social determinants of health [10,11,12,13,14]. This disparity was particularly evident early in the COVID-19 pandemic, driven by social determinants of health and barriers to care, such as lack of insurance, transportation, childcare, and occupational and housing conditions, as well as a higher prevalence of underlying medical co-morbidities among racial and ethnic minorities, ultimately leading to a higher risk of exposure to SARS-CoV2 and severe COVID-19 disease [21,22,23]. However, there is lack of research on the specific socio-economic factors associated with COVID-19 patients treated with RDV.
Given limited real-world data on socio-economic factors and RDV use, we evaluated clinical outcomes at our institution during the pre-vaccination period of the COVID-19 pandemic (primarily delta variant predominance), in Newark, New Jersey (NJ), serving a predominantly Black and Latino population, designated with a high CDC Social Vulnerability index (0.95) and COVID-19 Community Vulnerability Index (0.91) [24].
Methods
We conducted a retrospective study of adult patients (18 years and older) hospitalized with COVID-19 and who received at least one dose of RDV at University Hospital in Newark, NJ, between May 1, 2020, and April 30, 2021. Patients were excluded they received RDV prior to EUA on May 1, 2020, if their creatinine clearance was less than 30 mL/min, or if they had liver function tests over 10 times the upper limit of normal prior to FDA approval of RDV, and if they had prior hypersensitivity to RDV or any of its components. The study protocol was approved by the Institutional Review Board at Rutgers University.
Data were extracted from electronic medical records for patient demographics, comorbidities, disease severity, disease course, RDV administration, concomitant COVID-19 treatments, adverse events, and clinical outcomes. Social deprivation index (SDI) at the zip code level was obtained from the American Community Survey (ACS) [25]. The SDI is a composite measure of area level deprivation based on seven demographic characteristics collected in the ACS and used to quantify the socio-economic variation in health outcomes.
Disease severity and clinical outcomes were assessed on admission and hospital days 5, 7, 14, 28, or discharge (whichever came first), and were assessed according to the 8-point World Health Organization (WHO) Ordinal Scale for Clinical Improvement [26]. For each assessment, the highest category met on that day was recorded.
The primary outcome was mortality by day 28 or discharge, whichever came first. Secondary outcome was the proportion of patients who had recovered by day 14, defined as a score of 1, 2, or 3 on the ordinal scale.
Patient demographic and clinical characteristics were descriptively summarized using frequencies and proportions, or medians and interquartile ranges, as appropriate. Wilcoxon rank-sum tests (continuous variables) or Chi-square and Fisher’s exact tests (categorical variables) were used to compare demographic, socio-economic, and clinical factors by baseline WHO status and mortality by day 28. Among those with baseline WHO status 4 or greater, we compared demographic, socio-economic, and clinical factors among those who did and did not recover by day 14. All statistical analyses were performed using SAS version 9.4 (Cary, NC, USA).
Results
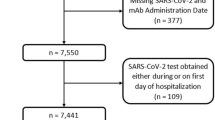
A total of 612 patients were hospitalized with COVID-19 and received 1 or more doses of RDV between May 1, 2020, and April 30, 2021. Of these, a convenience sample of 244 patients was randomly selected for chart review. Of those reviewed, 38 were excluded (29 had GFR < 30 ml/min, 3 had liver function tests > 10 times the upper limit of normal, 5 for positive SARS-Co-V-2 PCR outside of study period, and 1 patient with documented hypersensitivity to RDV component). The remaining 206 patients’ charts were included in the analysis.
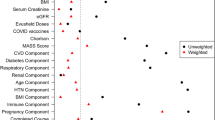
Baseline demographic and clinical characteristics of the study population are presented in Table 1, stratified by baseline WHO status of 3 or less versus WHO status 4 or greater. The median age of patients was 59 years and ranged from 19 to 96 years. Overall, 41% were non-Hispanic Black (NHB) and 42% were Hispanic. Approximately half (48%) were identified as female. Twenty-six percent were uninsured, 20% were Medicaid-insured, and 25% were Medicare-insured. The median SDI was 98. Prevalent baseline medical co-morbidities included hypertension (52%), diabetes (37%), hyperlipidemia (22%), asthma or COPD (20%), and coronary artery disease (10%). Many patients were overweight (30%) or obese (46%), with a median BMI of 29. A majority of the cohort (62%) had two or more underlying comorbid conditions.
Seventy percent of the study population met criteria of severe COVID-19 on admission by baseline O2 saturation, with 51% requiring oxygen support by mask or nasal cannula (meeting WHO ordinal scale criteria 4), 16% requiring noninvasive ventilation or high flow oxygen (meeting WHO ordinal scale criteria 5), and 3% required ventilatory support (meeting WHO ordinal scale criteria 6 and 7). Overall, 13% of patients were admitted to the ICU on admission. Of the 206 patients, (85%) received 3 or more days of RDV. Eighty-five percent of patients received systemic steroids. All patients except one were not fully vaccinated at time of their COVID-19 diagnosis.
Overall mortality at 28 days was 11% (23/206). Mortality was higher among those who were older (p = 0.01), those with underlying diabetes mellitus (p = 0.047), those with more severe illness on admission by WHO Ordinal Scale (WHO status 4 or more), and those on concomitant tociluzimab or convalescent plasma use (Table 2). Among 145 patients with baseline WHO status of 4 or greater, 35% recovered by day 5, 55% by day 7, 81% by day 14, and 86% by day 28. Fifteen percent did not recover by 28 days. Younger patients were more likely to recover by day 14 compared to older patients, though this was not statistically significant. Recovery by day 14 was inversely associated with older age and concomitant tociluzimab and convalescent plasma use (P < 0.001 and p = 0.001 respectively). Those with underlying diabetes and with HIV at baseline were less likely to recover by 14 days (Table 3).
RDV was generally tolerated. Only 5 patients had severe adverse events related to RDV. These included 2 with elevated liver function tests greater than 5 times the upper limit of normal, 2 with bradycardia and 1 related to phlebitis at the site of the intravenous line.
Discussion
In conclusion, our study provides valuable insights into the clinical outcomes of remdesivir treatment among patients hospitalized with COVID-19, particularly in a predominantly Black and Latino patient population with high social and community vulnerability indices (Social Vulnerability Index 0.95 and COVID-19 Community Vulnerability Index 0.91). Despite these challenging patient demographics, we observed that the overall mortality at 28 days in our cohort was comparable to previously reported randomized clinical trials with RDV and a large survey of hospitalized COVID-19 patients [15, 16]. In a survey with over 16,000 patients hospitalized for COVID-19 between March and December 2020, the overall mortality rate was 11%, ranging monthly from 7 to 17% [9]. Compared to death rates during the initial phase of the COVID-19 pandemic, COVID-19 mortality rates among hospitalized patients declined over the course of the pandemic, even before the introduction of wide-spread COVID-19 vaccination (which occurred after our study period). This decrease in mortality rates may be attributed to several factors, including improved clinical management of COVID-19, the development of new therapies, increased access to early treatment, and better resource allocation in institutional systems that were not overburdened.
The majority of patients (81%) who received RDV recovered within 7 days of RDV use, comparable to previously reported findings from RCTs. Several RCTs evaluating RDV use in hospitalized adult patients with COVID-19 showed RDV benefit in shortening time to clinical recovery (10 versus 15 days) [15, 16] or showed improved clinical status compared to standard of care [17]. However, RDV had no effect on 28-day mortality rates in these trials [15,16,17, 27].
We found that traditional risk factors, such as advanced age and multiple co-morbidities, particularly diabetes, was associated with increased risk for severe COVID-19 and death [1,2,3,4,5,6,7,8,9] as well as increased time to recovery. Data suggest that co-existence of COVID-19 with comorbidities, such as diabetes, cardiovascular disease, cancer, and others may lead to chronic inflammation, impaired immune response, and reduced host tolerance to organ injury, that may predispose to severe, potentially fatal COVID-19 [28, 29]. Worse outcomes associated with concomitant tocilizumab and convalescent plasma use likely reflected underlying severity of COVID presentation at baseline.
However, we acknowledge the limitations of our study, including its retrospective design and the absence of a control group, which may have influenced the interpretation of our results. As a result, the analyses were primarily descriptive, and the conclusions should be interpreted with caution considering these inherent limitations. Our study also highlights the importance of considering potential disparities in healthcare outcomes, as the limited number of non-White participants limited our capacity to fully assess the impact of race and other socio-economic factors on treatment outcomes.
Regarding safety, RDV was in general well-tolerated in our study, with few adverse events noted. Rare adverse events included abnormal liver function tests, and one case each of bradycardia, and peripheral line-associated phlebitis. These findings are consistent with previous safety assessments and provide reassurance about RDV’s acceptability profile.
Conclusion
In conclusion, our study provides valuable real-world evidence of RDV's outcomes among a vulnerable patient population, shedding light on its effectiveness in the context of socio-economically disadvantaged minorities. While our findings contribute to the understanding of RDV's clinical performance, further research with larger and more diverse patient cohorts, including controlled prospective studies, is essential to strengthen the evidence base and facilitate evidence-based decision-making in the ongoing battle against COVID-19. Additionally, efforts to address healthcare disparities and promote inclusive research practices will be critical in achieving better health outcomes for all patients affected by this devastating disease.
Data Availability
The data supporting this study are available by the corresponding author upon reasonable request.
References
Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 55700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9.
Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966.
Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775.
Williamson EG, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19 related death using OpenSAFELY. Nature. 2020;584(7821):430.
CDC COVID-19 Response Team. Severe outcomes among patients with Coronavirus Disease 2019 (COVID-19)- United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–6. https://doi.org/10.15585/mmwr.mm6912e2.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with Coronavirus Disease 2019-United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–6. https://doi.org/10.15585/mmwr.mm6913e2.
Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019 – COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wklly Rep. 2020;60(15):458–64.
Munoz-Price LS, Nattinger AB, Rivera F, et al. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Netw Open. 2020;3:e2021292.
Wortham JM, Lee JT, Althomsons S, et al. Characteristics of persons who died with COVID-19 – United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(28):923–9.
Yang X, Zhang J, Chen S, et al. Demographic disparities in clinical outcomes of COVID-19: data from a statewide cohort in South Carolina. Open Forum Infect Dis. 2021;8(9):ofab428.
Rossen LM, Branum AM, Ahmad FB, et al. Excess deaths associated with COVID-19, by age and race and ethnicity –United States, January 26-October 3. MMWR Morb Mortal Wkly Rep. 2020;69:1522–7.
Acosta A, Garg S, Pham H, et al. Racial and ethnic disparities in rates of COVID-19 -associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. JAMA Netw Open. 2021;4(10):e213-479.
Wang Y, Zhang D, Guanhua Du, et al. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–78.
Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383:1813–26.
Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57.
Remdesivir (package insert). Foster City, CA: Gilead Sciences, Inc; 2023. https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed 12 Jul 2023.
U.S. Food and Drug Administration. FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19-the science of safety and effectiveness. https://www.fda.gov/drugs/news-events-human-drugs/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness. Accessed 12 Jul 2023.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed 19 Jul 2023.
Goldman N, Pebley AR, Lee K, et al. Racial and ethnic differentials in COVID-19 related job exposures by occupational status in the US. medRxiv. https://www.medrxiv.org/content/10.1101/2020.11.13.20231431v2. Accessed 19 Jul 2023.
Hawkins D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am J Ind Med. 2020;63:817–20.
Debopadhaya S, Sprague AD, Mou H et al. Social determinants associated with COVID-19 mortality in the United States. https://www.medrxiv.org/content/10.1101/2020.08.23.20183848v2. Accessed 12 Jul 2023.
Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry/Geospatial Research, Analysis, and Services Program. CDC/ATSDR Social Vulnerability Index 2020 Database [NJ]. https://www.atsdr.cdc.gov/placeandhealth/svi/data_documentation_download.html. Accessed on July 1, 2023.
United States Census Bureau/American FactFinder. “B11001:household type (including living alone).” 2007–2011 American Community Survey. U.S. Census Bureau’s American Community Survey Office, 2011. Web. http://factfinder2.census.gov. Accessed on July 1, 2023.
World Health Organization. WHO R&D Blueprint Novel Coronavirus COVID-19 Therapeutic Trial Synopsis. 2020. https://www.who.int/blueprint/priority-diseaes/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020. Accessed 12 Jul 2023.
WHO Solidarity Trial Consortium. Repurposed anti-viral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med. 2021;384(6):497–511.
Bigdelou B, Sepan MR, Najafikhoshnoo S et al. COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes. Front Immunol. 13:8901517. https://doi.org/10.3389/fimmu.2022.890517.
Russell CK, Lone NI, Baillie JK. Comorbidities, multimorbidity and COVID-19. Nat Med. 2023;29:334–43. https://doi.org/10.1038/s41591-022-021.
Funding
This work was supported by the Gilead Sciences, Inc.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by Aparna Alankar, Malithi Desilva, Swetha Kodali, Tricia Mae Raquepo, Naema Meilad, Alexander Sudyn, Debra Chew, MD, MPH, and Shobha Swaminathan MD. Data analysis was performed by Sree Sudharshan and Stephanie Shiau, PhD, MPH. The first draft of the manuscript was written by Debra Chew, MD, MPH, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by our Institutional Review Board at Rutgers University.
Consent to Participate
Not applicable.
Consent to Publication
Not applicable.
Competing Interests
Dr. Shobha Swaminathan served as a consultant and is on the Speakers Bureau for Gilead Sciences. The remaining authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chew, D., Shiau, S., Sudharshan, S. et al. Outcomes Among Patients Hospitalized for COVID-19 Treated with Remdesivir in an Urban Center Pre-COVID-19 Vaccination. J. Racial and Ethnic Health Disparities (2023). https://doi.org/10.1007/s40615-023-01861-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40615-023-01861-6