Abstract
Background
Underrepresented racial and ethnic groups (UREGs) with HIV have a higher risk of cardiovascular disease (CVD) compared with the general population. Referral to a cardiovascular specialist improves CVD risk factor management in high-risk individuals. However, patient and provider factors impacting the likelihood of UREGs with HIV to have an encounter with a cardiologist are unknown.
Methods
We evaluated a cohort of UREGs with HIV and borderline CVD risk (10-year risk ≥ 5% by the pooled cohort equations or ≥ 7.5% by Framingham risk score). Participants received HIV-related care from 2014–2020 at four academic medical centers in the United States (U.S.). Adjusted Cox proportional hazards regression was used to estimate the association of patient and provider characteristics with time to first ambulatory cardiology encounter.
Results
A total of 2,039 people with HIV (PWH) and borderline CVD risk were identified. The median age was 45 years (IQR: 36–50); 52% were female; and 94% were Black. Of these participants, 283 (14%) had an ambulatory visit with a cardiologist (17% of women vs. 11% of men, p < .001). In fully adjusted models, older age, higher body mass index (BMI), atrial fibrillation, multimorbidity, urban residence, and no recent insurance were associated with a greater likelihood of an encounter with a cardiologist.
Conclusion
In UREGs with HIV and borderline CVD risk, the strongest determinants of a cardiology encounter were diagnosed CVD, insurance type, and urban residence. Future research is needed to determine the extent to which these encounters impact CVD care practices and outcomes in this population.
Trial Registration
ClinicalTrials.gov Identifier: NCT04025125.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
People with HIV (PWH) are at an increased risk for major adverse cardiovascular events, including myocardial infarction, heart failure, stroke, and sudden cardiac death [1,2,3]. In addition to biologic mechanisms, such as HIV-related chronic inflammation and antiretroviral-associated metabolic disorders, healthcare system, patient, and clinician factors are also implicated in the greater burden of cardiovascular disease (CVD) among PWH [4, 5]. These factors include, but are not limited to, differences in access to specialty care, treatment differences, and the lack of clear clinical guidelines for CVD risk management, which have all been associated with worse CVD care and outcomes among PWH compared with the general population [6,7,8]. This burden is particularly acute in the Southern United States (U.S.), a region that accounts for more than half of all incident HIV infections [9]. Underrepresented racial and ethnic groups (UREGs) and women with HIV in the Southern U.S. are disproportionately affected as these groups have a greater burden of CVD risk factors and poorer HIV and CVD outcomes[10,11,12].
Despite awareness of CVD prevention and risk reduction in PWH as national research and implementation priorities, there remains significant heterogeneity in how CVD risk factors are managed in PWH. These differences in CVD risk factor management are partially accounted for by clinician specialty and heterogeneity in the likelihood of HIV clinicians to refer patients to cardiology specialist care [13, 14]. For example, PWH receiving primary care services from their HIV provider are less likely to be prescribed guideline-indicated statin therapy than patients in an internal medicine clinic [15]. These challenges are compounded by sex-based differences in access to specialty care [16, 17]. Inclusion of a cardiovascular specialist in the care team for high-risk individuals has been shown to improve CVD risk factor management in the general population [18]. Unfortunately, UREG individuals and women are less likely to be referred to cardiologists compared with White patients and men, even those at high risk for CVD [19, 20].
We currently lack sufficient knowledge of the factors that impact cardiology specialist care for UREG PWH, a population disproportionately impacted by elevated CVD risk and poor CVD outcomes. This knowledge is crucial to develop evidence-based strategies to provide optimal and equitable CVD care for all PWH. Therefore, the objective of the Pathways to Cardiovascular Disease Prevention and Impact of Specialty Referral in Under-Represented Racial/Ethnic Minorities with HIV (PATHWAYS) study was to determine the likelihood of, and factors associated with, a cardiologist encounter among UREG PWH with borderline CVD risk. Given the observed underutilization of cardiologists for CVD care among women within the general population, our analysis also focused on sex-stratified risk determinants and encounters with specialists.
Methods
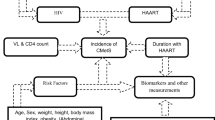
The PATHWAYS study (NCT04025125, https://clinicaltrials.gov/ct2/show/NCT04025125) is a multi-institutional collaborative observational study assessing the frequency of cardiology encounters for UREG PWH with borderline risk of atherosclerotic CVD (ASCVD) in the Southern U.S. Additionally, the study evaluates the multilevel (patient and clinical environment level) [21, 22] determinants associated with specialist encounters.
Patient population
We utilized patient-level data from the electronic health records (EHRs) collected retrospectively from selected institutions in the Stakeholders, Technology, and Research (STAR) Clinical Research Network (CRN) that had been harmonized into the National Patient-Centered Clinical Research Network (PCORnet) Common Data Model (CDM) [23]. The PCORnet CDM provides a standardized representation of common EHR data domains and is coupled with a data curation process to assess data quality. The institutions included Duke University (Durham, North Carolina), Vanderbilt University (Nashville, Tennessee), Medical University of South Carolina (Charleston, South Carolina), and Wake Forest Baptist Health (Winston-Salem, North Carolina).
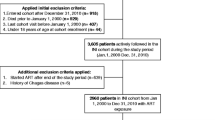
Figure 1 outlines how we applied the eligibility, inclusion, and exclusion criteria. Participants considered for inclusion were UREG patients retained in HIV care (defined by an HIV viral load laboratory test, a prescription for antiretroviral therapy [ART], and/or an encounter with an HIV provider in the 12 months prior to their index date) [1] at these institutions between January 1, 2014 and December 31, 2020. We defined UREGs as patients who self-reported as Black/African American, American Indian or Alaska Native, Asian, Multiple Race, or Hispanic who had confirmed HIV infection with any clinical contact at a participating site. We did not restrict our definition of UREG beyond race and ethnicity. We additionally restricted our analysis to persons with newly elevated ASCVD risk between January 1, 2015 and June 30, 2019 defined as: (1) age ≥ 40 years and a 10-year ASCVD risk ≥ 5% (calculated by the pooled cohort equation [PCE]) or ≥ 7.5% (calculated by the simplified Framingham risk score) [24,25,26], or (2) age < 40 years and a lifetime ASCVD risk ≥ 39%. We chose these thresholds to define a low to borderline risk group [27]. We defined the patient’s index date as the first date of elevated ASCVD risk, and we observed all cardiology encounters through December 31, 2020. Follow-up was censored if a patient died during the follow-up period without a cardiologist visit; the patient was followed to the end of the study (December 31, 2020) without a cardiology visit; or the patient’s last ambulatory visit occurred prior to the end of the study (censored at 6 months after the last follow-up).
Consort Diagram of the PATHWAYS Cohort. During the eligibility period (2014–2020), inclusion and exclusion criteria for HIV status, race and clinical contact were applied. We began the observation period in 2015 to allow for a 1-year lookback period to observe data needed to calculate ASCVD risk scores. The observation period ended in 2019 to allow for at least 1 year of observation through the end of 2020. Pre-existing ASCVD events included acute myocardial infarction, heart failure, acute coronary syndrome, stable or unstable angina, arterial revascularization (including coronary arterial or peripheral), stroke, transient ischemic attack, or atherosclerotic peripheral arterial disease based on ICD-9/10 codes. ASCVD, atherosclerotic cardiovascular disease
We then excluded patients who had an encounter with a cardiologist or an ASCVD event in the 12 months prior to their index date. Additionally, we excluded patients if they lacked ≥ 1 modifiable risk factor (ie, current smoker status, body mass index [BMI] > 25 kg/m2, or a diagnosis of diabetes mellitus, hypertension, or dyslipidemia as defined in the next section) assessed in the 12 months prior to their index date.
Variable definitions
The primary outcome for this analysis was time from index date to first outpatient encounter with a cardiology specialist. Data on initiation of a referral to a cardiologist were not available. The health care provider associated with a visit was classified based on the provider specialty listed in the CDM and the provider’s National Provider Identifier restricted to ambulatory clinic visits (see Supplementary Table 1). Encounters for cardiac diagnostic testing alone were not included as such testing could be ordered by any clinician. Baseline comorbidities were defined based on diagnoses coded on the index date or within 2 years prior. A full list of all diagnoses and codes applied for this study are in Supplementary Table 2. Blood pressure control was defined as the most recent systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mmHg among those with hypertension. Lipid control was defined as non-HDL cholesterol < 130 mg/dL among those with dyslipidemia. Baseline laboratory measures were based on the closest measurement from 2 years prior through 6 months after the index date to maximize data available to calculate CVD risk. Baseline medications were based on relevant prescriptions on the index date or within 13 months prior to account for prescriptions potentially written annually. Supplementary Table 3 contains the full list of medication concepts and drug names. Health insurance was determined from payers listed on the index encounter or within 13 months prior to the index encounter to maximize recorded insurance from infrequent clinic visits, and a patient could have multiple insurance types assigned (ie, Medicare/Medicaid, private, Ryan White HIV/AIDS Program [RWHAP], other, and none). The Charlson Comorbidity Index was determined using the method of Glasheen et al. [28]. Baseline viral suppression was defined as an HIV viral load < 200 copies/mL or undetectable. A patient was classified as having a history of AIDS if they had a CD4 + T-cell count < 200 cells/uL at any time within 2 years prior to through 6 months after the index date or had diagnoses associated with AIDS [28]. Clinical encounter visits separated by ≥ 7 days were considered unique episodes of care.
Statistical analysis
We described demographic, social, and clinical characteristics in the overall cohort and by sex. Continuous variables are described using their median and interquartile range (IQR). Categorical variables are described by their frequency (out of available sample size if missing values) and percentages. Comparisons between categories were performed with a Kruskal–Wallis test for continuous variables and a chi-squared test for categorical variables.
We calculated the cumulative percentage of PWH with a cardiology visit after their first elevation in ASCVD risk. Cox proportional hazards regression was used to characterize the univariable association of patient demographics, HIV status, medical history, CVD risk factors, and health care utilization with time to first cardiology visit. Hazard ratios with 95% confidence intervals (CIs) and p-values are provided for each univariable test.
To identify the subset of variables most strongly associated with time to first cardiology visit, we used multivariable Cox proportional hazards analysis with backward variable selection and p > 0.05 as the exit criteria. Baseline variables considered as candidates for inclusion in the model were identified a priori and included age, sex, insurance type, rural vs. urban residence, social deprivation index score, CD4 + T-cell count, viral suppression, ART prescription, atrial fibrillation, Charlson comorbidity score, estimated glomerular filtration rate (eGFR), BMI categories, blood pressure control (not hypertensive, hypertensive controlled, hypertensive not controlled), lipid control (not diagnosed hyperlipidemia, controlled hyperlipidemia, uncontrolled hyperlipidemia), current smoker status, and primary provider (HIV vs. primary care). Observations with missing values were excluded from descriptive and univariable analyses. For the multivariable modeling, multiple imputation (25 imputed datasets) was used to fill in missing covariate values (missingness summarized by sex in Supplementary Table 4). Models were run on each imputed dataset, and estimates were obtained by aggregating results across imputations. All p-values are two-sided, and a value < 0.05 is considered statistically significant. No adjustment was made for multiple comparisons. SAS V9.4 (SAS Institute, Cary, North Carolina) was used to perform the statistical analyses.
Institutional ethics review and approval
The Duke University Health System Institutional Review Board (IRB) served as the single IRB of record (Pro00101663, Pro00101104) with approval of a waiver of informed consent. Using a SMART IRB agreement, all sites relied on Duke for IRB review and approval. All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association.
Results
We identified 2039 individuals (median age: 45 years [IQR: 36–50], 52% female, 94% Black or African American) across the four institutions (Table 1). Women were on average 6 years older and had slightly greater BMI, systolic blood pressure, and diastolic blood pressure than men (all p < 0.001, Table 1). The median CD4 + T-cell count was 605 cells/uL (IQR: 378–858), and most participants (89%) had achieved viral suppression. ART use in the 2 years surrounding the date when eligibility was met was overall similar between the groups.
As Table 1 highlights, most individuals reported at least one encounter with no or missing insurance information during this period (58%). After that, Medicare/Medicaid (41%), private insurance (46%), and RWHAP (26%) were the most common insurance types. Supplementary Table 5 shows the cross-tabulations of insurance types, with insurance and clinical variables demonstrating no meaningful differences in CVD risk scores across insurance types. Most individuals reported multiple insurance types, suggesting changes in insurance coverage throughout the study period.
Overweight/obesity (76%), hypertension (38%), current smoker status (29%), and elevated total cholesterol (27%) were the most common CVD risk factors across the study population, which were also more common among women. The prevalence of diabetes was higher among women (14% vs. 4%, p < 0.001). Data to calculate CVD risk scores were available on a subset of participants (72% for ASCVD risk score, 95% for Framingham risk score, and 100% for lifetime risk score, see Supplementary Table 5). In participants ≥ 40 years old, ASCVD risk scores were greater among men, with 60% of men and 47% of women having an ASCVD risk score ≥ 5%. A similar pattern was seen for lifetime ASCVD risk score in participants < 40 years old, where the median score was 46% for men and 39% for women (Table 1). Lipid control was similar among both sexes, but women less often had blood pressure control than men (41% vs. 54%, p < 0.001).
Healthcare utilization patterns are shown in Table 2. Most individuals (57%) had an infectious disease specialist as their only primary care provider. After meeting eligibility criteria based on CVD risk threshold, 283 (14%) individuals had an ambulatory visit with a cardiologist. Women were more likely to have seen a cardiologist than men (17% vs. 11%, p < 0.001). Among participants seeing a cardiologist during our study period, the time interval between meeting the CVD risk threshold and an encounter with a cardiologist was a median of 24 months (20 months in women vs. 32 months for men). Through 60 months of follow-up, the cumulative percentage of participants who were seen by a cardiologist was higher among women than men (Supplementary Fig. 1). By the end of 60 months of follow-up, less than 25% of all individuals had a cardiology visit. The most common cardiovascular diagnoses associated with these visits were related to hypertension, chest pain, other potential cardiac symptoms, and hyperlipidemia (see Supplementary Table 6).
We next modeled the likelihood of an encounter with a cardiologist using both univariable and multivariable analyses. On univariable testing, older age was associated with a higher likelihood of seeing a cardiologist (HR: 1.48, 95% CI: 1.30–1.69), whereas male sex and rural residence were associated with a lower likelihood (HR: 0.60, 95% CI: 0.47–0.77; HR: 0.53, 95% CI: 0.31–0.89, respectively) (Table 3). Individuals who recently reported private, RWHAP, or other insurance types were almost 50% less likely to have an encounter with a cardiologist. Atrial fibrillation (HR: 5.32, 95% CI: 3.02–9.29), hypertension (HR: 1.59, 95% CI: 1.26–2.01), and current smoker status (HR: 1.36, 95% CI: 1.07–1.74) were among the strongest univariable predictors of an encounter with a cardiologist. Having an HIV specialist as a primary care provider (versus not) was associated with a greater likelihood of a cardiology encounter (HR: 1.63, 95% CI: 1.29–2.06), as was a greater number of ambulatory clinic visits per year (Table 3).
All variables included for selection in our multivariable modeling are shown in Table 4. Patients with a diagnosis of atrial fibrillation were over 3 times more likely to see a cardiologist (HR: 3.20, 95% CI: 1.81–5.66) in multivariate testing. Having at least one recent encounter with no or missing insurance was associated with twice the likelihood of seeing a cardiologist (HR: 2.11, 95% CI: 1.61–2.76). Older patients and those with a higher BMI and more comorbidities were also more likely to have an encounter with a cardiologist.
Discussion
Using real-world data, we examined the relationship between multidimensional factors and encounters with a cardiology specialist in a cohort of UREG PWH with borderline risk of ASCVD in the Southern U.S. In this cohort, we found that: (1) a small proportion of patients had subsequent encounters with a cardiologist after crossing ASCVD risk thresholds, and (2) the strongest determinants of an encounter with a cardiologist were related to a diagnosis of CVD, insurance type, and urban residence.
Our study is the first, to our knowledge, to report on the frequency of cardiology encounters for UREG PWH at borderline CVD risk. Access to specialty care is usually driven by referrals from a primary care physician. Most patients had an infectious disease specialist as their primary care provider, which was associated with a greater likelihood of seeing a cardiologist than having a primary care physician. This may be related to the reported discomfort among infectious disease specialists with managing CVD [29, 30]. In the HIV Outpatient Study, for example, less than 50% of hypertensive PWH being seen at HIV specialty clinics were treated according to prevailing guidelines [31]. Similar to the current analysis, men and those with private insurance were less likely to meet treatment guidelines. Okeke et al. also demonstrated worse compliance with hypertension treatment guidelines when comparing PWH treated in HIV clinics to primary care offices [32]. Our findings, however, may suggest a shift in care patterns in response to the growing attention to CVD risk management in PWH. While the effect of clinician specialty was attenuated in multivariate modeling, more research is needed to understand the relevant provider and patient factors related to more effective linkage to specialty care [29].
Patient and provider characteristics have repeatedly been shown to impact care for CVD. We are aware of no other published studies that have addressed the expected frequency of cardiology encounters for this borderline risk group with HIV. Prior work related to the pattern of cardiology referrals for individuals without manifest CVD has focused on care patterns for individuals with non-specific chest pain in primary care clinics or in non-U.S. health care systems [16, 33,34,35]. Cook et al. showed that women had less access to cardiologists than men despite a similar burden of CVD [16]. In a German cohort of primary care practices, for example, 14.5% of patients eventually had a cardiology encounter over a period of 1 year [33]. Our study is unique in its focus on UREG PWH and on individuals without known CVD. Given the doubling of cardiovascular events in PWH compared with rates estimated by current cardiovascular disease risk calculators, we suggest that a higher rate than observed in our study (13.9%) may be reasonable clinically [36]. While overall rates of encounters with cardiology are unexpectedly low, we found that UREG women had more and earlier visits with a cardiologist after meeting our ASCVD risk threshold than men. Sex differences were attenuated in the multivariate analysis perhaps due to the large difference in age between men and women in this cohort.
Health insurance is a critical component of access to appropriate healthcare in the U.S. for PWH [37]. In the current analysis, reporting “no insurance” on at least one recent encounter was strongly linked to a subsequent encounter with a cardiologist. While this may seem counterintuitive on the surface, the nuances of how healthcare is covered for PWH offers some potential explanations. The RWHAP is the “payer of last resort” for PWH who meet state-dependent income thresholds to cover costs related to HIV care and other medical comorbidities [38]. RWHAP fee schedules and formularies differ based on state and local policies [39]. Due to the constraints imposed by a lack of traditional health insurance, HIV clinic staff must often find innovative ways to assist under- or uninsured patients. For example, McManus et al. highlighted that when one state’s RWHAP program ended its coverage for CVD risk factor management, clinic staff were instrumental in finding alternative sources of coverage and CVD risk factor care was preserved despite the apparent lack of insurance coverage [40]. Webel et al. have also shown that sponsor-supported services substantially shape preventative CVD care in HIV clinics in North Carolina and Ohio [41].
Individual institutions also vary greatly in how coverage for services are recorded in the EHR. Specifically, patients classified as having “no insurance” at our participating sites could either be: (1) truly uninsured with limited access to providers due to cost constraints, (2) uninsured but met income criteria for institutional charitable programs that waived out-of-pocket expenses for basic access to subspecialty providers and encounters, or (3) in a state where RWHAP covered subspecialty services, but administratively the EHR did not recognize such coverage as insurance. We posit that the strong linkage we observed between reporting no insurance and seeing a cardiologist is likely due to institutional efforts to link patients to specialty care using non-traditional methods, HIV clinic reorganization to manage patient’s comorbid medical conditions, and innovative support programs, similar to previous studies [42, 43].
The strengths of this analysis include a study population from a real-world setting enriched for UREG PWH. Several limitations are also worth noting. The scope of data was largely limited to elements currently collected via the CDM, which largely represents information captured in structured fields within the EHR, although we did also use complementary stand-alone approaches to extract elements, such as encounters with specialists. Data on initiation of a referral to a cardiologist were not available. We chose the point at which an individual’s CVD risk crossed a particular threshold and linked this time point to future visits with a cardiologist. Because it is possible that provider’s awareness of crossing this threshold may have been later in time, we opted for a long follow-up period to observe future cardiology visits. We excluded individuals with prior ASCVD events, which limits generalizability and may introduce detection bias as it relates to the prevalence estimates of risk factors. Exposures, such as insurance type or others, are susceptible to misclassification due to the nature of these EHR data. In addition, only clinical data related to encounters within these health systems were included, and we may have missed encounters with primary care providers or cardiologists outside of these systems, such as in community-based clinics. Lastly, in the absence of a consensus on risk score thresholds for cardiology referrals among PWH, we used elevated and borderline risk thresholds based on guidelines for the general population [27].
In summary, in UREG PWH with borderline CVD risk, clinical, geographic, and socio-economic factors are associated with subsequent encounters with a cardiologist. These factors represent potential directed targets to increase use of cardiology specialty care for UREG PWH. The relationship between cardiology specialty care and subsequent CVD events in this population warrants further research.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request and will be made publicly available through the National Institutes of Health.
References
Freiberg MS, Chang CCH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. https://doi.org/10.1001/jamainternmed.2013.3728.
Shah ASV, Stelzle D, Lee KK, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with the Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis. Circulation. Published online July 2, 2018:CIRCULATIONAHA.117.033369. https://doi.org/10.1161/CIRCULATIONAHA.117.033369
Erqou S, Jiang L, Choudhary G, et al. Heart Failure Outcomes and Associated Factors Among Veterans With Human Immunodeficiency Virus Infection. JACC Heart Fail. 2020;8(6):501–11. https://doi.org/10.1016/j.jchf.2019.12.007.
So-Armah K, Freiberg MS. HIV and Cardiovascular Disease: Update on Clinical Events, Special Populations, and Novel Biomarkers. Curr HIV/AIDS Rep. 2018;15(3):233–44. https://doi.org/10.1007/s11904-018-0400-5.
So-Armah K, Freiberg MS. Cardiovascular disease risk in an aging HIV population: not just a question of biology. Curr Opin HIV AIDS. 2014;9(4):346–54. https://doi.org/10.1097/COH.0000000000000065.
Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55(11):1550–7. https://doi.org/10.1093/cid/cis752.
Pearce D, Ani C, Espinosa-Silva Y, et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol. 2012;110(8):1078–84. https://doi.org/10.1016/j.amjcard.2012.05.045.
Womack JA, Chang CCH, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3(5):e001035. https://doi.org/10.1161/JAHA.114.001035
Centers for Disease Control and Prevention (CDC). HIV in the United States by Region: HIV Incidence.; 2022. https://www.cdc.gov/hiv/statistics/overview/incidence.html
Kent ST, Schwartz JE, Shimbo D, et al. Race and sex differences in ambulatory blood pressure measures among HIV+ adults. J Am Soc Hypertens. 2017;11(7):420-427.e3. https://doi.org/10.1016/j.jash.2017.05.002.
Ramírez-Marrero FA, De Jesús E, Santana-Bagur J, Hunter R, Frontera W, Joyner MJ. Prevalence of cardiometabolic risk factors in Hispanics living with HIV. Ethn Dis. 2010;20(4):423–8.
Richardson KK, Bokhour B, McInnes DK, et al. Racial Disparities in HIV Care Extend to Common Comorbidities: Implications for Implementation of Interventions to Reduce Disparities in HIV Care. J Natl Med Assoc. 2016;108(4):201-210.e3. https://doi.org/10.1016/j.jnma.2016.08.001.
Shah MR. The Broad Spectrum of HIV-Related Cardiovascular Disease. JACC: Heart Failure. 2015;3(8):600–602. https://doi.org/10.1016/j.jchf.2015.04.007
Walensky RP, Auerbach JD, Office of AIDS Research Advisory Council, et al. Focusing National Institutes of Health HIV/AIDS Research for Maximum Population Impact. Clin Infect Dis. 2015;60(6):937–940. https://doi.org/10.1093/cid/ciu942.
Okeke NL, Chin T, Clement M, Chow SC, Hicks CB. Coronary artery disease risk reduction in HIV-infected persons: a comparative analysis. AIDS Care. 2016;28(4):475–82. https://doi.org/10.1080/09540121.2015.1099602.
Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119(18):2463–70. https://doi.org/10.1161/CIRCULATIONAHA.108.825133.
Dale SK, Dean T, Sharma R, Reid R, Saunders S, Safren SA. Microaggressions and Discrimination Relate to Barriers to Care Among Black Women Living with HIV. AIDS Patient Care STDS. 2019;33(4):175–83. https://doi.org/10.1089/apc.2018.0258.
Gilstrap LG, Malhotra R, Peltier-Saxe D, et al. Community-Based Primary Prevention Programs Decrease the Rate of Metabolic Syndrome Among Socioeconomically Disadvantaged Women. Journal of Women’s Health. 2013;22(4):322–9. https://doi.org/10.1089/jwh.2012.3854.
Navar AM, Wang TY, Li S, et al. Lipid management in contemporary community practice: Results from the Provider Assessment of Lipid Management (PALM) Registry. American Heart Journal. 2017;193:84–92. https://doi.org/10.1016/j.ahj.2017.08.005.
Schulman KA, Berlin JA, Harless W, et al. The Effect of Race and Sex on Physicians’ Recommendations for Cardiac Catheterization. New England Journal of Medecine. 1999;340(8):618–26. https://doi.org/10.1056/NEJM199902253400806.
Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748.
Mehrotra A, Forrest CB, Lin CY. Dropping the baton: specialty referrals in the United States. Milbank Q. 2011;89(1):39–68. https://doi.org/10.1111/j.1468-0009.2011.00619.x.
Qualls LG, Phillips TA, Hammill BG, et al. Evaluating Foundational Data Quality in the National Patient-Centered Clinical Research Network (PCORnet®). EGEMS (Wash DC). 2018;6(1):3. https://doi.org/10.5334/egems.199.
Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. https://doi.org/10.1016/j.jacc.2013.11.005.
Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. New England Journal of Medecine. 2012;366(4):321–9. https://doi.org/10.1056/NEJMoa1012848.
D’Agostino RBS, Grundy S, Sullivan LM, Wilson P, Group CHDRP. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187.
Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98–124. https://doi.org/10.1161/CIR.0000000000000695.
Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am Health Drug Benefits. 2019;12(4):188–97.
Muiruri C, Corneli A, Cooper L, et al. Perspectives of HIV specialists and cardiologists on the specialty referral process for people living with HIV: a qualitative descriptive study. BMC Health Services Research. 2022;22(1):623. https://doi.org/10.1186/s12913-022-08015-0.
Fultz SL, Goulet JL, Weissman S, et al. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clin Infect Dis. 2005;41(5):738–43. https://doi.org/10.1086/432621.
Lichtenstein KA, Armon C, Buchacz K, et al. Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Prev Chronic Dis. 2013;10:E10. https://doi.org/10.5888/pcd10.120083.
Okeke NL, Schafer KR, Meissner EG, et al. Cardiovascular Disease Risk Management in Persons With HIV: Does Clinician Specialty Matter? Open Forum Infect Dis. 2020;7(9):ofaa361. https://doi.org/10.1093/ofid/ofaa361
Glombiewski JA, Rief W, Bösner S, Keller H, Martin A, Donner-Banzhoff N. The course of nonspecific chest pain in primary care: symptom persistence and health care usage. Arch Intern Med. 2010;170(3):251–5. https://doi.org/10.1001/archinternmed.2009.474.
Rask KJ, Deaton C, Culler SD, et al. The effect of primary care gatekeepers on the management of patients with chest pain. Am J Manag Care. 1999;5(10):1274–82.
Buch V, Ralph H, Salas J, Hauptman PJ, Davis D, Scherrer JF. Chest Pain, Atherosclerotic Cardiovascular Disease Risk, and Cardiology Referral in Primary Care. J Prim Care Community Health. 2018;9:2150132718773259. https://doi.org/10.1177/2150132718773259.
Soares C, Kwok M, Boucher KA, et al. Performance of Cardiovascular Risk Prediction Models Among People Living With HIV: A Systematic Review and Meta-analysis. JAMA Cardiol. 2023;8(2):139–49. https://doi.org/10.1001/jamacardio.2022.4873.
Jabs AW, Jabs DA, Van Natta ML, Palella FJ, Meinert CL, Studies of the Ocular Complications of AIDS Research Group. Insurance status and mortality among patients with AIDS. HIV Med. 2018;19(1):7–17. https://doi.org/10.1111/hiv.12531
National Alliance of State and Territorial AIDS Directors (NASTAD). 2021–2022 National RWHAP Part B and ADAP Monitoring Project Report. Accessed February 14, 2022. https://nastad.org/partb-adap-2021-2022-report
Blackstock OJ, Wang KH, Fiellin DA. State variation in AIDS drug assistance program prescription drug coverage for modifiable cardiovascular risk factors. J Gen Intern Med. 2011;26(12):1426–33. https://doi.org/10.1007/s11606-011-1807-5.
McManus KA, Rodney RC, Rhodes A, Bailey S, Dillingham R. Affordable Care Act Qualified Health Plan Enrollment for AIDS Drug Assistance Program Clients: Virginia’s Experience and Best Practices. AIDS Research Human Retroviruses. 2016;32(9):885–91. https://doi.org/10.1089/AID.2016.0033.
Webel AR, Schexnayder J, Rentrope CR, et al. The influence of healthcare financing on cardiovascular disease prevention in people living with HIV. BMC Public Health. 2020;20(1):1768. https://doi.org/10.1186/s12889-020-09896-8.
Mandsager P, Marier A, Cohen S, Fanning M, Hauck H, Cheever LW. Reducing HIV-Related Health Disparities in the Health Resources and Services Administration’s Ryan White HIV/AIDS Program. Am J Public Health. 2018;108(S4):S246–50. https://doi.org/10.2105/AJPH.2018.304689.
Fix GM, Asch SM, Saifu HN, Fletcher MD, Gifford AL, Bokhour BG. Delivering PACT-principled care: are specialty care patients being left behind? J Gen Intern Med. 2014;29 Suppl 2(S2):S695–702. https://doi.org/10.1007/s11606-013-2677-9
Acknowledgements
We acknowledge Mr. Paul Hofmann for creating the analysis datasets and support from the Stakeholders, Technology and Research Clinical Research Network (STAR CRN).
Funding
Research reported in this publication was supported by the National Institute of Minority Health and Health Disparities (R01MD013493, PI: Bloomfield); the National Institutes of Health (Okeke, PI: K23HL137611-04; R01MH113438, PI: Pettit); the National Institute of General Medical Sciences (P20GM130457, PI: Meissner); and the Tennessee Center for AIDS Research (P30AI110527, PI: Pettit).
Author information
Authors and Affiliations
Contributions
Concept and design: Bloomfield, Hill, Chiswell, Longenecker, Meissner, Thomas, Pettit, Okeke.
Acquisition, analysis, or interpretation of data: Bloomfield, Hill, Chiswell, Cooper, Gray, Longenecker, Louzao, Marsolo, Meissner, Morse, Muiruri, Thomas, Velazquez, Vicini, Pettit, Sanders, Okeke.
Drafting of the manuscript: Bloomfield, Hill, Chiswell, Sanders, Okeke.
Critical revision of the manuscript for important intellectual content: Bloomfield, Hill, Chiswell, Cooper, Gray, Longenecker, Marsolo, Meissner, Morse, Muiruri, Thomas, Velazquez, Vicini, Pettit, Okeke.
Statistical analysis: Hill, Chiswell.
Obtained funding: Bloomfield.
Administrative, technical, or material support: Sanders.
Corresponding author
Ethics declarations
Competing Interests
Dr. Lance Okeke reports consulting fees from Gilead Sciences. Dr. Keith Marsolo reports grants and contracts to his institution from Novartis, Amgen, Seqirus, Genentech, BMS, and Boehringer Ingelheim. Dr. Eric G. Meissner serves on an expert panel for Viiv Healthcare. The other authors report no conflicts of interest relevant to this article.
Ethics Approval
The Duke University Health System Institutional Review Board (IRB) served as the single IRB of record (Pro00101663, Pro00101104) with approval of a waiver of informed consent. Using a SMART IRB agreement, all sites relied on Duke for IRB review and approval. All procedures followed were in accordance with the ethical standards of the Declaration of Helsinki of the World Medical Association.
Consent to Participate
The Duke University Health System IRB approved a waiver of informed consent.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bloomfield, G.S., Hill, C.L., Chiswell, K. et al. Cardiology Encounters for Underrepresented Racial and Ethnic Groups with Human Immunodeficiency Virus and Borderline Cardiovascular Disease Risk. J. Racial and Ethnic Health Disparities 11, 1509–1519 (2024). https://doi.org/10.1007/s40615-023-01627-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-023-01627-0