Abstract
Purpose of Review
In this article, we aim to provide an overview of the occurrence and characteristics of livestock-associated (LA-) meticillin-resistant Staphylococcus aureus (MRSA). We further question the role of LA-MRSA as a potential foodborne pathogen. We investigate recent findings and developments from a One Health perspective also highlighting current strategies and initiatives aiming to improve reporting, control, and prevention of LA-MRSA.
Recent Findings
While the overall number of invasive MRSA infections in humans is decreasing (in most European countries and the USA) or steadily increasing (in the Asia-Pacific region), the role of LA-MRSA as causative agent of invasive disease and as potential foodborne pathogen is still poorly understood. LA-MRSA prevalence in livestock remains high in many geographical regions and the acquisition of new virulence and resistance determinants constitutes a growing threat for human health.
Summary
The true incidence of LA-MRSA infections due to occupational exposure is unknown. Improved MRSA monitoring and tracking procedures are urgently needed. Strain typing is crucial to enable improved understanding of the impact of LA-MRSA on human and animal health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Livestock-associated meticillin-resistant Staphylococcus aureus (LA-)MRSA has gained particular interest since the first findings of LA-MRSA of clonal complex CC398/sequence type (ST)398 in pigs in 2005 in France [1•] and The Netherlands [2]. Soon thereafter, CC398 was also identified in other animals (including dairy cattle, veal calves, poultry, dogs, cats, and horses) and it became clear that LA-MRSA should be considered a zoonosis [2] with people with occupational contact with livestock (e.g., farmers, veterinarians, and workers at abattoirs) being frequently exposed and often colonized. Since LA-MRSA CC398 is able to cause the same kind of infections in humans as S. aureus and MRSA in general, severe infections in people further indicated that the animal reservoir of S. aureus can have serious consequences for human health [3, 4•, 5, 6••].
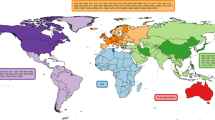
LA-MRSA evolved independently from common hospital-acquired (HA) or community-associated (CA) MRSA usually found in humans [7•], and mainly belong to S. aureus clonal complex CC398 and associated spa types t011, and t034 [5]. CC398 shows a broader host range compared to other MRSA strains and has been detected in cattle, veal calves, horses, poultry, companion animals (dogs and cats), horses, and in humans [8]. However, also other CCs such as CC1, CC97, CC130, and CC5 are found in livestock around the globe [9]. CC398 remains the most commonly identified type of LA-MRSA in most European countries. However, while MRSA CC398 strains have been found in livestock across the globe [10], a different strain of LA-MRSA, CC9, appears to be the prominent type in several Asian countries [11]. In the USA, the diversity of LA-MRSA appears to be higher than in Europe or Asia, with reports of both CC398 and a variety of “human” types of S. aureus in livestock [12]. For an overview of the different CCs and STs of LA-MRSA detected in different animal hosts and geographical regions, see Fig. 1.
Usually, CC398 MRSA carry resistance genes to a variety of antimicrobial classes such as tetracycline, macrolides, or aminoglycosides [13,14,15] and tend to exhibit increased levels of multiple drug resistances compared with non-CC398 MRSA strains [16]. Further it was postulated that the presence of the resistance determinants tetK/tetM and czrC confers a fitness advantage [17••]. The meticillin-resistant gene mecA is found in LA-MRSA strains, but mecC, a gene variant sharing 70% identity at the DNA level with mecA, has been detected in ruminants, pigs, and companion animals, with increasing reports from wild animals [18, 19]. MRSA-ST398 isolates usually possess a bunch of different virulence-associated factors such as hemolysins and immune-modulatory factors, but MRSA ST398 commonly lack Panton-Valentine leukocidin (PVL) or staphylococcal enterotoxins (SE) [20], of which a significant number is located on mobile genetic elements, e.g. temperate phages [21]. In contrast, strains of CC9 commonly harbor enterotoxin genes and tst-1 encoding the toxic shock syndrome toxin [11].
LA-MRSA can be found in foods intended for human consumption. The contamination sources for foods, especially those from animal origins such as raw meat and associated products, may be livestock as well as humans involved in animal husbandry and food processing. The presence of MRSA in/on food intended for human consumption may not necessarily define MRSA as a foodborne pathogen [22]. Moreover, foodborne outbreaks due to meticillin-resistant strains are not expected to be more severe than those caused by meticillin-susceptible strains as the severity of the intoxication is not directly related to the antimicrobial resistance profile of the causative S. aureus strain [23]. Nevertheless, it was recently proposed by the European Food Safety Authority (EFSA) to reinforce the monitoring of MRSA in food-producing animals as well as in food [24].
In this article, we review the current situation of MRSA in the different livestock species such as pigs, dairy cattle and veal calves, poultry, and other (non-farm) animals. We further address the findings of LA-MRSA in food and question, whether LA-MRSA has to be considered a foodborne pathogen. Finally, we discuss all these recent findings and developments from a One Health perspective and present approaches to improve detection, control, and prevention of LA-MRSA.
MRSA in Livestock
In the following section, we will summarize the most recent findings with regard to MRSA in livestock. In doing so, we would like to emphasize that the prevalence data presented for the various animal species may not necessarily be comparable with each other due to differing sampling and isolation methods used. But It is however out of the scope of this review to present and discuss on the sensitivity and/or specificity of the diagnostic methods applied in the different studies.
MRSA in Pigs
All around the globe, LA-MRSA are frequently detected along the entire pork production chain from primary pig production to pig meat, with predominant lineages changing over time and differing between geographical regions [11, 25, 26]. Pigs are the primary host of LA-MRSA CC398, with the first report dating back to 2005 [27]. LA-MRSA CC398 have since become widespread across pig breeding and production farms all over Europe, with prevalence rates varying substantially among different European countries: by using a harmonized monitoring approach (collecting dust samples in farms, nasal samples from individual pigs, and a standardized isolation method [24]). The EFSA reported between 0% and 90% positive animals/herds/batches in 2017 and 0% and 89% positive herds in 2018, respectively [28]. Interestingly, following highly successful eradication programs, Norway reported a prevalence of 0.4% in 2017 and no positive pig herds in 2018 [28]. Pig movements between farms are considered an important driver in the spread of LA-MRSA CC398 [17••]. Recently, a Danish study compared MRSA herd prevalence in fattening pigs raised under controlled housing conditions (conventional indoor fattening pigs) to those raised under non-controlled housing conditions (free-range fattening pigs including organic production herds). The prevalence in herds raised conventionally (89%) substantially exceeded the prevalence in free-range herds (20%) [28]. Similar to Europe, the prevalence of LA-MRSA in pigs in Asian countries varies widely among different geographical regions. Different studies report a prevalence of 1% in Japan and Malaysia, 3% in South Korea, 11% in China, 10-40% in Thailand, 16-39% in Hong Kong, and 4-43% in Taiwan [11].
The MRSA burden on pig carcasses can be significantly reduced by hygienic measures during the slaughter process, in particular by scalding and flaming [25]. Still, recontamination can occur either through contaminated equipment or through human handling during further meat processing [25]. In order to minimize transmission of MRSA from pig primary production to pork, optimizing decontamination during the slaughter processes as shown by Lassok et al. [25] and avoiding recontamination by effective cleaning and high hygiene standards for personnel are crucial.
An MRSA CC398 strain detected in Finnish fattening pigs [26] lacked both the global virulence regulator gene locus agr and the adhesion gene fnbB. The strain was shown to exhibit increased adhesive capacity to human and porcine host cells and diminished cytotoxic effects on porcine host cells, characteristics that are likely favorable for persistent colonization in pigs, as well as for transmission to and among human hosts [26].
MRSA in Dairy Cattle
In dairy cattle, S. aureus is considered a contagious mastitis pathogen that enters the mammary gland through the teat canal. Staphylococcus aureus is among the common pathogens causing clinical mastitis to date [29], if not the most prevalent pathogen isolated from mastitis milk samples [30]. Meticillin-resistant S. aureus has been reported from dairy farms around the world and associated with transmission events between human and animals, but the directionality of transmission is not always known [31]. Overall, the MRSA prevalence in dairy herds is low compared to other animal species, mainly ranging from 0.0 to 4.4% MRSA-positive bulk tank milk samples [32, 33•]. However, there is some evidence that the MRSA prevalence might be increasing over time at least in some countries, e.g. from 4.4 (year 2009-2010) to 9.7% (year 2014) in Germany [34], and up to 6% (until 2006) and 13.9% (in 2011-2012) in Korea [35].
As in pigs, the predominant MRSA type in dairy herds in Europe is LA-MRSA of clonal complex 398. Also, in studies from Brazil, China, and Israel, LA-MRSA CC398 were found in mastitis milk samples [32]. Very recently, LA-MRSA exclusively belonging to CC398 were found on 20 German dairy farms [33•]. In contrast, ST9 MRSA were found in milk samples in Southeast Asia [36]. In most studies, a transmission from cow to cow was suggested as there is usually a predominant MRSA strain within herds [32]. However, the environment may also act as a reservoir of MRSA strains found in dairy herds, too [37]. Hansen et al. [38•] were the first who suggested a spillover of LA-MRSA from pigs into cattle farms based on their phylogenetic analysis applying whole-genome sequencing.
Several risk factors for the occurrence of MRSA in dairy herds were identified. MRSA was more frequently detected in conventional dairy farms than in organic farms and more often in larger farms than in smaller farms; pigs and humans introducing new MRSA strains into dairy herds were identified as additional risk factors [32]. Within dairy herds, transmission was associated with improper milking hygiene procedures [33•, 37] and somatic cell counts of bulk tank milk were elevated on MRSA-affected farms [33•]. Additionally, meticillin-resistant coagulase-negative staphylococci may transfer resistance genes to S. aureus prevalent in dairy but this needs to be further investigated [32]. In contrast, the debate about the role of antimicrobial exposure as a risk factor for the occurrence of MRSA in dairy cows is still ongoing. Resistance levels through intra-mammary treatment might be lower than in other parts of the body after oral or parenteral application of antibiotics [39].
Clonal MRSA strains might be widely spread among different animals and the milking equipment, as described recently [33•, 40•]. Moreover, MRSA transmission between different farms has been observed [40•]. However, since isolates found on dairy farms lacked factors typically associated with human infection, the risk for severe human infections and foodborne diseases was considered to be low [40•]. Also, Hansen and colleagues [38•] concluded that Danish cattle represent a low prevalence reservoir for LA-MRSA CC398 which might not be of major human health concern.
MRSA in Veal Calves
Besides pigs, veal calves remain the main reservoir for LA-MRSA in livestock partly with increasing prevalence in European countries over the years [28]. Compared to other farms raising bovines, i.e. dairy or beef cattle farms, MRSA are mainly present in veal farms with CC398-associated spa types predominating [38•, 41, 42]. Calves were more often MRSA carriers when treated with antibiotics [43, 44]. In contrast, farm hygiene contributes to lower prevalence of MRSA and a 2-3 times lower prevalence of MRSA was also observed in calves younger than 6 weeks of age [45•, 46]. On the contrary, Graveland and colleagues [46] hypothesized that antimicrobial use may not necessarily be the only risk factor for MRSA in veal calves. Applying DNA microarray and PCR for detection of antimicrobial resistance and virulence genes, Argudin and colleagues [47] found that meticillin-resistant non-S. aureus from veal calves represents an important reservoir of antimicrobial resistance and virulence genes. Recently, the results of one of the first whole-genome sequencing approaches on LA-MRSA were published and a possible spillover from pig production into veal calf farming was postulated [38•].
MRSA in Poultry
Although the prevalence of MRSA in poultry is lower compared to the prevalence in pigs and cattle [28, 48], MRSA have been detected along the whole poultry production chain from farm to fork. In a recent systematic review, a meta-analytical method was used to estimate the “pooled prevalence” of MRSA [49•]. Ribeiro and colleagues (2018) established the MRSA prevalence from 51 studies published from 2003 to 2017, assessed the heterogeneity, and calculated the “pooled MRSA prevalence” by using the random effects model. The MRSA prevalence rates in poultry and poultry meat varied between geographical regions, with highest MRSA prevalence being observed in South America (27%) and lowest MRSA prevalence being observed in North America (1%) [49•].
MRSA isolates from poultry belonged to various clonal lineages. In Europe, the main lineage detected in broiler chicken and chicken retail meat products was CC398, but CC5, CC8, CC9, and CC80 were also isolated from poultry [50,51,52,53,54,55]. A study from Belgium for the first time reported MRSA isolates assigned to ST398 (spa types t011 and t567) that were obtained from poultry in 2006 [53]. A study screening for MRSA in laying hens and broiler chickens in Belgium in 2007 detected MRSA CC398 of spa type t1456 in broiler chickens, which differs from spa types detected in other animal species in Belgium and Europe [54]. In addition, no MRSA were detected in laying hens [54]. A Canadian study investigating LA-MRSA isolates from chicken meat and broiler flock samples (2013-2014) reported a prevalence of 1.3% in chicken meat samples and no positive samples from broiler chickens [52]. All isolates were assigned to ST398 and ST8 [52]. In recent years, the number of studies providing data on the population structure and evolution of poultry MRSA was very low, which may result in changes in predominant clones going unnoticed.
MRSA in Other Animals
LA-MRSA has been detected not only in livestock, but also in other animals regularly found on farms, i.e. goats, cats, dogs, mice, and rats [33•, 56]. In companion, animals such as cats and dogs [33•, 57, 58] as well as in horses [33•, 58,59,60,61], LA-MRSA, mainly CC398, plays an important role. Particularly in horses, CC398 MRSA are massively prevalent worldwide and represent nearly 90% of the MRSA isolated from equine wound infections in Germany [57]. Previous surgery, hospitalization, treatment with antimicrobial agents, treatment contact with human MRSA carriers, and use of implant devices are regarded as risk factors for MRSA infection in companion animals [62].
In regions with high livestock density, MRSA belonging to livestock-associated clonal lineages were exclusively found in companion animals, emphasizing the adverse effects of dissemination of multi-drug-resistant organisms (MDRO) across species barriers [63]. Kaspar and colleagues [63] further concluded that the presence of LA-MRSA among pets and probable dissemination in clinical settings supports the necessity of a One Health approach to address the potential threats due to MDRO-carrying companion animals. Also, in non-hospitalized horses living in rural areas, LA-MRSA belonging to CC398 were present, which underlines the impact of livestock on the geographic distribution of epidemic strains [63].
MRSA in Food
While there is a wealth of literature on the role of S. aureus as a foodborne pathogen [64], the role of MRSA in food is still poorly understood. The EFSA reported that voluntary monitoring of foods, healthy animals, and clinical investigations revealed that > 95% of the detected spa types were associated with LA-MRSA lineages [28]. Since carcasses can be contaminated during slaughter through contact with the skin, respiratory secretions, feces, urine, and other exudates, a possible route of dissemination of LA-MRSA to humans is through the food production chain. Cross-contamination and recontamination of the bacteria during food preparation, or consumption of meat, which was not properly cooked, may play an important role in the dissemination of LA-MRSA, and therefore could contribute to a serious health problem, especially for immunocompromised people. Therefore, monitoring of MRSA from farm-to-fork as well as the comparison of strains from livestock and food with those from humans remains highly recommended at European Union level. Recently, the EFSA has even proposed to reinforce the monitoring of MRSA in food-producing animals and food [24]. This may also include the characterization of MRSA isolates by genotypic analysis (whole-genome sequencing) to determine the phylogeny as well as to investigate the presence of important virulence and host-adaption factors and those specific genetic markers associated with certain animal hosts [24].
Many studies conducted in Europe and North America have confirmed the contamination of food, mainly raw meat, with LA-MRSA, sometimes with substantially high prevalence in poultry meat [65,66,67]. Newest prevalence data are available at EU level with MRSA prevalence ranging from 1 to 20% in broiler meat and from 43 to 100% in turkey meat, respectively [28]. Thus, one may assume that also LA-MRSA may act as foodborne pathogens as recently reviewed by Sergelidis and coauthors [23]; however, this depends on the staphylococcal enterotoxin (SE) gene content of LA-MRSA strains and requires favorable conditions for growth and enterotoxin production. Those clonal lineages present in the farm to fork food chain do not or only at a very minor percentage carry SE encoding genes [13]. In addition, the level of contamination of food may be low, as recently shown by Pauly and colleagues [68•] providing quantitative data on MRSA prevalence in fresh broiler meat samples for the first time.
On the other hand, LA-MRSA are relatively rare in urban areas and some cases of LA-MRSA carriage in humans cannot be explained by livestock contact. In The Netherlands, human carriers of CC398 MRSA of unknown origin carried MRSA from livestock origin, suggestive of indirect transmission [69]. Also, Deiters and colleagues [70] have speculated that humans might have acquired LA-MRSA via contaminated food. Adding to that, poultry meat, mainly from turkey, has been considered a probable source of infections in humans with a novel hybrid LA-MRSA CC9/CC398 genotype [71•]. As suggested by Larsen and colleagues [72•], specific LA-MRSA subpopulations such as CC9/CC398 might have become adapted to humans and might therefore more easily transferable via food.
In general, the risk of exposure to MRSA through consumption of contaminated food appears to be small in comparison with that related to the contact with livestock animals or humans. Very recently, it was shown in a probabilistic model approach that the prevalence of MRSA at retail level highly influences the probability of the final serving to be contaminated [73•]. Overall, the probability and extent of cross-contamination and recontamination and the burden of MRSA from contaminated raw chicken meat via hands and kitchen utensils during a household barbecue was low (i.e., the probability of the consumer to be exposed by at least one cell while consuming a serving would be smaller than 1.07 × 10−5 in 95% of the simulations) [73•]. Also, occupational handling of raw meat and raw meat products was not associated with an increased risk of nasal colonization by LA-MRSA [74•]. Nevertheless, it is important to keep good hygiene practices during the household food manipulation to reduce the spread of MRSA and any other bacteria.
Livestock-Associated MRSA in Humans and Its Impact From a One Health Perspective
It is scientific consensus that colonization and subsequent infections with LA-MRSA can occur in people who have direct contact with livestock. This affects, for example, farmers, veterinarians, or slaughterhouse employees. A direct association between animal and human carriage of CC398 MRSA was shown in pig [2] and veal calf farming [44]. Likewise, in the poultry production system, slaughterhouse staff in contact with live animals exhibited particularly high prevalence rates of MRSA carriage, in particular workers hanging broilers on the slaughter line (20%) [55]. The stunning technique chosen also influences the risk of LA-MRSA carriage in employees, with an increase in risk if conventional electric stunning was employed compared to CO2 stunning [55]. In veal calf farming, the environmental contamination with MRSA plays also a role in the acquisition of MRSA in veal calf farmers and their household members [75]. The exposure to CC398 MRSA in barn air seems to be an important determinant for nasal carriage in humans, especially in highly exposed veal calf farmers as well as duration of contact with animals [76].
The role of LA-MRSA in human invasive infections is difficult to assess. EU member states report data on antimicrobial susceptibility of invasive human S. aureus isolates to the European Antimicrobial Resistance Surveillance Network (EARS-Net) hosted by the European Centre for Disease Prevention and Control (ECDC). However, as typing data are not included in reporting, potential links between LA-MRSA animal reservoirs and infections in humans are currently not captured by these monitoring procedures. Between 2015 and 2018, the EARS-Net reporting data show a decrease in the population-weighted mean proportion of MRSA strains among invasive S. aureus infections from 19 to 16% [28]. However, the EFSA still considers MRSA a major threat for human health due to high MRSA levels in several countries and concerns with regard to combined resistance to other antimicrobial groups [28]. Monitoring systems in the USA are less centralized, with Centers for Disease Control and Prevention (CDC) and Centers for Medicare and Medicaid Services relying on National Healthcare Safety Network Reports, the Emerging Infections Program, and electronic health records. Still, similar to European reports, a trend towards a decline in human MRSA bloodstream infections was observed and largely attributed to decreasing numbers of HA-MRSA infections with strain USA100 [77, 78]. However, the role of LA-MRSA infections remain unclear. While in Europe and the USA there is a trend towards a decline in human MRSA infections, this might not be the case at global level. At least for the Asia-Pacific region, a steadily increasing trend since the 1980s was shown with regional detection proportions of meticillin resistance in S. aureus in healthcare settings ranging from 26 to 73% in 2011 [79].
LA-MRSA assigned to CC398 most likely originated in humans as meticillin-susceptible S. aureus [7•]. While the jump from humans to livestock was followed by acquisition of resistance to tetracycline and meticillin, it may have led to decreased capacity for human colonization and transmission [7•]. Very recently it was shown that LA-MRSA CC398 originated in the late 1990s and diversified into farm-specific genotypes, which stay relatively consistent over time [80]. However, the ability of MRSA-ST398 to acquire mobile genetic elements was reported to be increased [81]. Kraushaar and colleagues [82•] have already demonstrated that lysogenic conversion of LA-CC398 strains by virulence-associated phages may occur in vitro and that new pathotypes may emerge by this mechanism. In vivo, CC398 MRSA strains harboring pvl are of particular concern and first cases of hospitalizations and death have been reported [83,84,85•, 86]. Interestingly, two human cases without contact to animals or people working or living with animals were reported in patients in Tokyo, Japan [85•, 86]. The cases were caused by a PVL-positive LA-MRSA CC398 (ST1232) clone, a single-locus variant of ST398.
The directionality of transmission between livestock and humans has been a topic of controversy and there is some evidence for transmission from humans to animals, e.g. spread of healthcare-associated MRSA from farmers to pigs [87]. However, spread of LA-MRSA from animals to humans seems to occur frequently, as supported by a recent systematic review [88••] that demonstrated a monotonically increasing relationship for hours of livestock exposure. This systematic review also associated livestock exposure with an elevated risk of MRSA carriage (OR = 7.03, 95% CI 4.29-11.52) in general [88••]. Odds ratios varied significantly between animal species and exposed groups (see Tables 1 and 2) [88••]. In a study executed in Spain in 2016, 58% of pig farm workers were carriers of LA-MRSA CC398, compared to an MRSA prevalence rate of <0.5% in the general population [90••]. Another recent systematic review also stresses the role of occupational livestock contact and in particular pig contact enhancing the risk of LA-MRSA colonization [89]. The odds ratio for LA-MRSA colonization among swine workers was highest (OR = 15.41), followed by cattle workers (OR = 11.62), veterinarians (OR = 7.63), horse workers (OR = 7.45), poultry workers (OR = 5.70), and industrial slaughterhouse workers (OR = 4.69). The results of a systematic review by Klous and coworkers [87] suggest that the prevalence of MRSA carriage in slaughterhouse workers also has a spatial component: Prevalence rates in workers at the start of the slaughter line working with live animals exceed those of staff working with carcasses only. In contrast, nasal LA-MRSA colonization among humans with occasional livestock contact is common but most likely only temporary [80].
MRSA carriage has long been known to present a risk factor for MRSA infections, in particular in patients undergoing surgery [91]. However, the true incidence of LA-MRSA infections due to occupational exposure is unknown and would necessitate longitudinal studies of high-risk occupational groups that are currently not available. In addition, developing effective preventive strategies to minimize the risk of MRSA infections in persons in occupational contact with livestock is further complicated due to persistence and recolonization. A study repeatedly screening Dutch veterinarians for LA-MRSA colonization over a period of 2 years found that 44% of veterinarians were LA-MRSA carriers at one or more measurement time points, with 13% being persistently colonized with the same strain [92]. Decolonization strategies are available and often rely on a combination of topical use of mupirocin nasal spray or ointment and body washes with chlorhexidine, octenidine, or polyhexanide [93, 94•]. Approximately 95% of LA-MRSA are susceptible to mupirocin, but no similar data is available assessing the effectiveness of chlorhexidine, octenidine, or polyhexanide in vitro [94•]. In addition, though decolonization has clear advantages, e.g. in MRSA carriers undergoing planned surgery, long-term decolonization success rates are low in persons with continuous livestock exposure [94•].
Conclusion
Undoubtedly, LA-MRSA adds to the total burden of MRSA in humans. Reports of LA-MRSA strains acquiring new virulence and resistance determinants and causing severe bloodstream infections in humans are a major cause of concern. According to Gebreyes and colleagues [95•], more than two-thirds of emerging infectious diseases in humans today are known to be of animal origin. Understanding the origin, risk factors, transmission, prevention, and control of LA-MRSA strains has been a challenge for various reasons, particularly due to the intertwined nature of the human, environment, and animal health sectors. Food animal farms, pets in communities, slaughterhouses, and veterinary hospital environments are major sources of LA-MRSA infections. However, attributing such infections to a source requires comprehensive monitoring programs using highly discriminatory molecular methods such as whole-genome sequencing (WGS) or Fourier-transform infrared spectroscopy [96,97,98]. In particular, WGS-based approaches will increasingly be used in the near future and will allow for a better understanding of the molecular epidemiology of the diseases at the interface of humans, animals, and the environment; this may in part even result in the blurring of epidemiological classifications [4•]. The results will make a substantial contribution to the development of more suitable control and mitigation strategies which have to consider a One Health approach.
Change history
30 August 2021
Springer Nature’s version of this paper was updated to correct the Figure 1 caption.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Armand-Lefevre L, Ruimy R, Andremont A. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg Infect Dis. 2005;11:711–4.
van Loo I, Huijsdens X, Tiemersma E, de Neeling A, van de Sande-Bruinsma N, Beaujean D, et al. Emergence of methicillin resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13:1834–9.
Cuny C, Wieler LH, Witte W. Livestock-associated MRSA: the impact on humans. Antibiotics. 2015;4:521–43.
Bal AM, Coombs GW, Holden MTG, Lindsay JA, Nimmo GR, Tattevin P, et al. Genomic insights into the emergence and spread of international clones of healthcare-, community- and livestock associated meticillin-resistant Staphylococcus aureus: blurring of the traditional definitions. J Glob Antimicrob Resist. 2016;6:95–101 Bal: Interesting read questioning traditional definitions of HA-MRSA, CA-MRSA and LAMRSA.
Goerge T, Lorenz MB, van Alen S, Hübner NO, Becker K. KöckR. MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol. 2017;200:6–12.
Gajdács M. The continuing threat of methicillin-resistant Staphylococcus aureus. Antibiotics. 2019;8:52. Available from: https://pubmed.ncbi.nlm.nih.gov/31052511. Current overviewof the history and emergence of MRSA, as well as diagnostic and therapeutic advances.
Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio. 2012;3 A milestone in providing first insights into host adaptation and emergence of CC398 LAMRSA.
Verkade E, Kluytmans J. Livestock-associated Staphylococcus aureus CC398: animal reservoirs and human infections. Infect Genet Evol Elsevier BV. 2014;21:523–30.
Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol. 2012;20:192–8.
Smith TC. Livestock-associated Staphylococcus aureus: the United States experience. PLoS Pathog. 2015;11:e1004564.
Chuang Y-Y, Huang Y-C. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue? Int J Antimicrob Agents. Netherlands. 2015;45:334–40.
Chon J, Sung K, Khan S. Methicillin-resistant Staphylococcus aureus (MRSA) in food-producing and companion animals and food products. In: Enany S, Alexander LEC, editors. Front Staphylococcus aureus [Internet]. Rijeka: IntechOpen; 2017. Available from: https://doi.org/10.5772/66645
Kraushaar B, Ballhausen B, Leeser D, Tenhagen B-A, Käsbohrer A, Fetsch A. Antimicrobial resistances and virulence markers in Methicillin-resistant Staphylococcus aureus from broiler and turkey: a molecular view from farm to fork. Vet Microbiol. Netherlands. 2017;200:25–32.
Sharma M, Nunez-Garcia J, Kearns AM, Doumith M, Butaye PR, Argudín MA, et al. Livestock-associated methicillin resistant Staphylococcus aureus (LA-MRSA) clonal complex (CC) 398 isolated from UK animals belong to European lineages. Front Microbiol. 2016;7:1741.
Sørensen AIV, Toft N, Boklund A, Espinosa-Gongora C, Græsbøll K, Larsen J, et al. A mechanistic model for spread of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) within a pig herd. PLoS One. 2017;12:e0188429.
Back SH, Eom HS, Lee HH, Lee GY, Park KT, Yang SJ. Livestock-associated methicillin-resistant Staphylococcus aureus in Korea: antimicrobial resistance and molecular characteristics of LA-MRSA strains isolated from pigs, pig farmers, and farm environment. J Vet Sci. 2020;21:e2.
Sieber RN, Skov RL, Nielsen J, Schulz J, Price LB, Larsen AR, et al. Drivers and dynamics of methicillin-resistant livestock-associated Staphylococcus aureus CC398 in pigs and humans in Denmark. MBio. 2018;9:1–12 Novel and highly interesting insights into LA-MRSA drivers and dynamics.
Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22:42–7.
Bengtsson B, Persson L, Ekström K, Unnerstad HE, Uhlhorn H, Börjesson S. High occurrence of mecC-MRSA in wild hedgehogs (Erinaceus europaeus) in Sweden. Vet Microbiol Netherlands. 2017;207:103–7.
Argudín MA, Mendoza MC, González-Hevia MA, Bances M, Guerra B, Rodicio MR. Genotypes, exotoxin gene content, and antimicrobial resistance of Staphylococcus aureus strains recovered from foods and food handlers. Appl Environ Microbiol. 2012;78:2930–5.
Alibayov B, Baba-Moussa L, Sina H, Zdeňková K, Demnerová K. Staphylococcus aureus mobile genetic elements. Mol Biol Rep [Internet]. 2014;41:5005–5018. Available from: https://doi.org/10.1007/s11033-014-3367-3.
Wendlandt S, Schwarz S, Silley P. Methicillin-resistant Staphylococcus aureus: a food-borne pathogen? Annu Rev Food Sci Technol. United States. 2013;4:117–39.
Sergelidis D, Angelidis AS. Methicillin-resistant Staphylococcus aureus: a controversial food-borne pathogen. Lett Appl Microbiol. 2017;64:409–18.
Aerts M, Battisti A, Hendriksen R, Kempf I, Teale C, Tenhagen B-A, et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA Journal Eur Food Saf Auth. 2019;17:e05709.
Lassok B, Tenhagen B-A. From pig to pork: methicillin-resistant Staphylococcus aureus in the pork production chain. J Food Prot United States. 2013;76:1095–108.
Heikinheimo A, Johler S, Karvonen L, Julmi J, Fredriksson-Ahomaa M, Stephan R. New dominant spa type t2741 in livestock-associated MRSA (CC398-MRSA-V) in Finnish fatteningpigs at slaughter. Antimicrob Resist Infect Control. Antimicrob Resist Infect Control. 2016;5:1–6.
Voss A, Loeffen F. Bakker J. Staphylococcus aureus in pig farming. 2005;11:2004–5.
EFSA. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA Journal Eur Food Saf Auth. 2020;18:e06007.
Ruegg PL. Making antibiotic treatment decisions for clinical mastitis. Vet Clin North Am Food Anim Pract United States. 2018;34:413–25.
Osteras O Animal health and welfare: results of the IDF questionnaire Data obtained between 2014 and 2016 [Internet]. 2018. Available from: https://www.fil-idf.org/wp-content/uploads/2018/09/IDF-Animal-Health-Report-Web.pdf
Juhász-Kaszanyitzky E, Jánosi S, Somogyi P, Dán A, van der Graaf-van Bloois L, van Duijkeren E, et al. MRSA transmission between cows and humans. Emerg Infect Dis. 2007;13:630–2.
Schnitt A, Tenhagen B-A. Risk factors for the occurrence of methicillin-resistant Staphylococcus aureus in Dairy herds: an update. Foodborne Pathog Dis. 2020;17:585–96.
Schnitt A, Lienen T, Wichmann-Schauer H, Cuny C, Tenhagen BA. The occurrence and distribution of livestock-associated methicillin-resistant Staphylococcus aureus ST398 on German dairy farms. J Dairy Sci United States. 2020;103:11806–19 Overview of occurrence of LA-MRSA in dairy farms in Germany.
Tenhagen B-A, Alt K, Pfefferkorn B, Wiehle L, Käsbohrer A, Fetsch A. Short communication: methicillin-resistant Staphylococcus aureus in conventional and organic dairy herds in Germany. J Dairy Sci United States. 2018;101:3380–6.
Song JW, Yang SJ, Shin S, Seo KS, Park YH, Park KT. Genotypic and phenotypic characterization of methicillin-resistant Staphylococcus aureus isolated from bovine mastitic milk in Korea. J Food Prot United States. 2016;79:1725–32.
Wang X-M, Zhang W-J, Schwarz S, Yu S, Liu H, Si W, et al. Methicillin-resistant Staphylococcus aureus ST9 from a case of bovine mastitis carries the genes cfr and erm(A) on a small plasmid. J Antimicrob Chemother. England. 2012:1287–9.
Locatelli C, Cremonesi P, Caprioli A, Carfora V, Ianzano A, Barberio A, et al. Occurrence of methicillin-resistant Staphylococcus aureus in dairy cattle herds, related swine farms, and humans in contact with herds. J Dairy Sci. United States. 2017;100:608–19.
• Hansen JE, Ronco T, Stegger M, Sieber RN, Fertner ME, Martin HL, et al. LA-MRSA CC398 in dairy cattle and veal calf farms indicates spillover from pig production. Front Microbiol. 2019;10:2733 Spillover of LA-MRSA from pig production into other production animals raises concern with regard to human exposure.
Lam T, Scherpenzeel C, den Uijl I, van Schaik G. Dry cow therapy–does it still deserve a blanket recommendation. nNMC. Annu Meet. 2014;2014:14.
Lienen T, Schnitt A, Hammerl JA, Maurischat S, Tenhagen B-A. Genomic distinctions of LA-MRSA ST398 on dairy farms from different German federal states with a low risk of severe human infections. Front Microbiol. 2020;11:575321 Very current genomic insights into LA-MRSA from dairy farms.
Tenhagen B-A, Vossenkuhl B, Käsbohrer A, Alt K, Kraushaar B, Guerra B, et al. Methicillin-resistant Staphylococcus aureus in cattle food chains-prevalence, diversity, and antimicrobial resistance in Germany. J Anim Sci. United States. 2014;92:2741–51.
Nemeghaire S, Argudín MA, Haesebrouck F, Butaye P. Epidemiology and molecular characterization of methicillin resistant Staphylococcus aureus nasal carriage isolates from bovines. BMC Vet Res. 2014;10:153.
Bos MEH, Graveland H, Portengen L, Wagenaar JA, Heederik DJJ. Livestock-associated MRSA prevalence in veal calf production is associated with farmhygiene, use of antimicrobials, and age of the calves. Prev Vet Med. Netherlands. 2012;105:155–9.
Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E, Heederik D. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS One. 2010;5:e10990.
Dorado-García A, Dohmen W, Bos MEH, Verstappen KM, Houben M, Wagenaar JA, et al. Dose-response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg Infect Dis. 2015;21:950–9 Interesting insights into the dose-response relationship between antimicrobial use and porcine LA-MRSA.
Graveland H, Wagenaar JA, Verstappen KMHW, Oosting-van Schothorst I, Heederik DJJ, Bos MEH. Dynamics of MRSA carriage in veal calves: a longitudinal field study. Prev Vet Med. Netherlands. 2012;107:180–6.
Argudín MA, Vanderhaeghen W, Butaye P. Diversity of antimicrobial resistance and virulence genes in methicillin-resistant non-Staphylococcus aureus staphylococci from veal calves. Res Vet Sci. England. 2015;99:10–6.
Huber H, Koller S, Giezendanner N, Stephan R, Zweifel C. Prevalence and characteristics of meticillin-resistant Staphylococcus aureus in humans in contact with farm animals, in livestock, and in food of animal origin, Switzerland, 2009. Eurosurveillance. 2010;15.
Ribeiro CM, Stefani LM, Lucheis SB, Okano W, Cruz JCM, Souza GV, et al. Methicillin-resistant Staphylococcus aureus in poultry and poultry meat: a meta-analysis. J Food Prot. 2018;81:1055–62 One of the rare meta-analyses available for MRSA. This one is focusing on MRSA associated with poultry.
Stegger M, Lindsay JA, Moodley A, Skov R, Broens EM, Guardabassi L. Rapid PCR detection of Staphylococcus aureus clonal complex 398 by targeting the restriction-modification system carrying sau1-hsdS1. 2011;49:732–4.
Feßler AT, Kadlec K, Hassel M, Hauschild T, Eidam C, Ehricht R, et al. Characterization of methicillin-resistant Staphylococcus aureus isolates from food and food products of poultry origin in Germany. Appl Environ Microbiol. 2011;77:7151–7.
Bernier-Lachance J, Arsenault J, Usongo V, Parent É, Labrie J, Jacques M, et al. Prevalence and characteristics of livestock associated methicillin-resistant Staphylococcus aureus (LAMRSA) isolated from chicken meat in the province of Quebec Canada. PLoS One. 2020;15:e0227183.
Nemati M, Hermans K, Lipinska U, Denis O, Deplano A, Struelens M, et al. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: first detection of livestock-associated methicillin-resistant strain ST398. Antimicrob Agents Chemother. 2008;52:3817–9.
Persoons D, Van Hoorebeke S, Hermans K, Butaye P, de Kruif A, Haesebrouck F, et al. Methicillin-resistant Staphylococcus aureus in poultry. Emerg Infect Dis. 2009;15:452–3.
Mulders MN, Haenen APJ, Geenen PL, Vesseur PC, Poldervaart ES, Bosch T, et al. Prevalence of livestock-associated MRSA in broiler flocks and risk factors for slaughterhouse personnel in The Netherlands. Epidemiol Infect. 2010;138:743–55.
Pletinckx LJ, Verhegghe M, Crombé F, Dewulf J, De Bleecker Y, Rasschaert G, et al. Evidence of possible methicillin-resistant Staphylococcus aureus ST398 spread between pigs and other animals and people residing on the same farm. Prev Vet Med. Netherlands. 2013;109:293–303.
Vincze S, Stamm I, Kopp PA, Hermes J, Adlhoch C, Semmler T, et al. Alarming proportions of methicillin-resistant Staphylococcus aureus (MRSA) in wound samples from companion animals. Germany 2010–2012. 2014;9:1e85656.
Schmitt S, Stephan R, Huebschke E, Schaefle D, Merz A, Johler S. DNA microarray-based characterization and antimicrobial resistance phenotypes of clinical MRSA strains from animal hosts. J Vet Sci. 2020;21:1–11.
Cuny C, Witte W. MRSA in equine hospitals and its significance for infections in humans. Vet Microbiol. Netherlands. 2017;200:59–64.
Islam MZ, Espinosa-Gongora C, Damborg P, Sieber RN, Munk R, Husted L, et al. Horses in Denmark are a reservoir of diverse clones of methicillin-resistant and -susceptible Staphylococcus aureus. Front Microbiol. 2017;8:543.
Haenni M, Châtre P, Dupieux-Chabert C, Métayer V, Bes M, Madec J-Y, et al. Molecular epidemiology of methicillin resistant Staphylococcus aureus in horses, cats, and dogs over a 5-year period in France. Front Microbiol. 2017;8:2493.
Loeffler A, Pfeiffer DU, Lindsay JA, SoaresMagalhães RJ, Lloyd DH. Prevalence of and risk factors for MRSA carriage in companion animals: a survey of dogs, cats and horses. Epidemiol Infect. England. 2011;139:1019–28.
Kaspar U, von Lützau A, Schlattmann A, Roesler U, Köck R, Becker K. Zoonotic multidrug-resistant microorganisms among small companion animals in Germany. PLoS One. 2018;13:e0208364.
Fetsch A, Johler S. Staphylococcus aureus as a foodborne pathogen. Curr Clin Microbiol Reports. 2018;5:88–96.
de Boer E, Zwartkruis-Nahuis JTM, Wit B, Huijsdens XW, de Neeling AJ, Bosch T, et al. Prevalence of methicillin-resistant Staphylococcus aureus in meat. Int J Food Microbiol. Netherlands. 2009;134:52–6.
Weese JS, Avery BP, Reid-Smith RJ. Detection and quantification of methicillin-resistant Staphylococcus aureus (MRSA) clones in retail meat products. Lett ApplMicrobiol. England. 2010;51:338–42.
Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL). Zoonoses Monitoring 2018-Summary findings and conclusions [Internet]. Available from: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/04_Zoonosen_Monitoring/Zoonosen_Monitoring_Bericht_2018_en-summary.pdf?__blob=publicationFile&v=3.
Pauly N, Wichmann-Schauer H, Ballhausen B, Torres Reyes N, Fetsch A, Tenhagen B-A. Detection and quantification of methicillin-resistant Staphylococcus aureus in fresh broiler meat at retail in Germany. Int J FoodMicrobiol. Netherlands. 2019;292:8–12 This paper is of particular interest, as it does not only present data on detection and quantification, but also a comparison of isolation methods.
Lekkerkerk WSN, van Wamel WJB, Snijders SV, Willems RJ, van Duijkeren E, Broens EM, et al. What is the origin of livestock associated methicillin-resistant Staphylococcus aureus clonal complex 398 isolates from humans without livestock contact? An epidemiological and genetic analysis. J Clin Microbiol. 2015;53:1836–41.
Deiters C, Günnewig V, Friedrich AW, Mellmann A, Köck R. Are cases of methicillin-resistant Staphylococcus aureus clonal complex (CC) 398 among humans still livestock-associated? Int J Med Microbiol. Germany. 2015;305:110–3.
Fetsch A, Kraushaar B, Käsbohrer A, Hammerl JA. Turkey meat as source of CC9/CC398 methicillin-resistant Staphylococcus aureus in humans? Clin Infect Dis. 2017:102–3 Rare data on CC9/CC398 MRSA from turkey meat strongly suggesting a role in human infections.
Larsen J, Stegger M, Andersen PS, Petersen A, Larsen AR, Westh H, et al. Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2016;63:1349–52 Evolutionary insights were gained by comparing WGS of CA-MRSA ST398 isolates to LAMRSA and methicillin-sensitive ST398.
Plaza-Rodríguez C, Kaesbohrer A, Tenhagen B-A. Probabilistic model for the estimation of the consumer exposure to methicillin resistant Staphylococcus aureus due to cross-contamination and recontamination. Microbiologyopen. 2019;8:e900 The role of MRSA cross-contamination and recontamination of meat during household food preparation was elucidated using a probabilistic model.
Cuny C, Layer F, Hansen S, Werner G, Witte W. Nasal colonization of humans with occupational exposure to raw meat and to raw meat products with methicillin-susceptible and methicillin resistant Staphylococcus aureus. Toxins (Basel). 2019;11 The authors present interesting data from a cross-sectional study on nasal S. aureus/MRSA colonization of butchers, meat sellers, and cooks in Germany.
Dorado-García A, Bos ME, Graveland H, Van Cleef BA, Verstappen KM, Kluytmans JA, et al. Risk factors for persistence of livestock-associated MRSA and environmental exposure in veal calf farmers and their family members: an observational longitudinal study. BMJ Open. 2013;3:e003272.
Bos MEH, Verstappen KM, van Cleef BAGL, Dohmen W, Dorado-García A, Graveland H, et al. Transmission through air as a possible route of exposure for MRSA. J Expo Sci Environ Epidemiol. United States. 2016;26:263–9.
Centers for Disease Control and Prevention. Healthcare associated infections-community-interface surveillance report, Emerging Infections Program Network methicillin-resistant Staphylococcus aureus. 2017;2020.
See I, Mu Y, Albrecht V, Karlsson M, Dumyati G, Hardy DJ, et al. Trends in incidence of methicillin-resistant Staphylococcus aureus bloodstream infections differ by strain type and healthcare exposure, United States, 2005–2013. Clin Infect Dis. 2020;70:19–25.
Lim WW, Wu P, Bond HS, Wong JY, Ni K, Seto WH, et al. Determinants of methicillin-resistant Staphylococcus aureus (MRSA) prevalence in the Asia-Pacific region: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2019;16:17–27.
Effelsberg N, Udarcev S, Müller H, Kobusch I, Linnemann S, Boelhauve M, et al. Genotypic characterization of livestock associated methicillin-resistant Staphylococcus aureus isolates of clonal complex 398 in pigsty visitors: transient carriage or persistence? J Clin Microbiol. 2019;58.
Schijffelen MJ, Boel CHE, Van Strijp JAG, Fluit AC. Whole genome analysis of a livestock-associated methicillin-resistant Staphylococcus aureus ST398 isolate from a case of human endocarditis. 2010
Kraushaar B, Hammerl JA, Kienöl M, Heinig ML, Sperling N, Dinh Thanh M, et al. Acquisition of virulence factors in livestock associated MRSA: lysogenic conversion of CC398 strains by virulence gene-containing phages. Sci Rep. 2017;7:2004 The presented data suggests that lysogenic conversion of LA-MRSA CC398 strains occurs and may lead to emergence of new pathotypes.
Welinder-Olsson C, Florén-Johansson K, Larsson L, Oberg S, Karlsson L, Ahrén C. Infection with Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus t034. Emerg Infect Dis. 2008;14:1271–2.
Koyama H, Sanui M, Saga T, Harada S, Ishii Y, Tateda K, et al. A fatal infection caused by sequence type 398 methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin gene: a case report in Japan. J Infect Chemother [Internet] Elsevier Ltd. 2015;21:541–543. Available from: http://linkinghub.elsevier. com/retrieve/pii/S1341321X15000847.
Nakaminami H, Hirai Y, Nishimura H, Takadama S, Noguchi N. Arthritis caused by MRSA CC398 in patient without animal contact Japan. Emerg Infect Dis. 2020;26:3104–5 Clinical case of arthritis caused by an MRSA of CC398 in a patient without livestock contact.
Nakaminami H, Kawasaki H, Takadama S, Kaneko H, Suzuki Y, Maruyama H, et al. Possible dissemination of a Panton-Valentine leukocidin-positive livestock-associated methicillin-resistant Staphylococcus aureus CC398 clone in Tokyo. Japan. Jpn J Infect Dis Japan. 2021;74:82–4.
Klous G, Huss A, Heederik DJJ, Coutinho RA. Human–livestock contacts and their relationship to transmission of zoonotic pathogens, a systematic review of literature. One Heal The Authors. 2016;2:65–76.
Liu Y, Han C, Chen Z, Guo D, Ye X. Relationship between livestock exposure and methicillin-resistant Staphylococcus aureus carriage in humans: a systematic review and dose response meta-analysis. Int J Antimicrob Agents. Elsevier B.V. 2020;55:105810 Highly interesting systematic review on livestock exposure and MRSA carriage in humans that also provides insights into dose-response relationships.
Reynaga E, Navarro M, Vilamala A, Roure P, Quintana M, Garcia-Nuñez M, et al. Prevalence of colonization by methicillin-resistant Staphylococcus aureus ST398 in pigs and pig farm workers in an area of Catalonia, Spain. BMC Infect Dis BMC Infectious Diseases. 2016;16:1–8.
Chen C, Wu F. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) colonisation and infection among livestock workers and veterinarians: a systematic review and meta-analysis. Occup Environ Med. 2020;0:1–11 Most current systematic review on LA-MRSA infections and carriage in persons with occupational contact to livestock.
Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–20.
Verkade E, van Benthem B, Kluytmans-van den Bergh M, van Cleef B, van Rijen M, Bosch T, et al. Dynamics and determinants of Staphylococcus aureus carriage in livestock veterinarians: a prospective cohort study. Clin Infect Dis. 2013;57:11–7.
Johler S, Nüesch-Inderbinen MT, Nüesch H, Hächler H, Stadler U, Stephan R. Intrafamilial spread of a Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus belonging to the paediatric clone ST5 SSCmec IV. JMM Case Reports. 2014;1:1–4.
Goerge T, Lorenz MB, van Alen S, Hübner N-O, Becker K, Köck R. MRSA colonization and infection among persons with occupational livestock exposure in Europe: prevalence, preventive options and evidence. Vet Microbiol. Elsevier B.V. 2017;200:6–12 Overview of occupation-related infections due to LA-MRSA CC398, including their incidence and preventive strategies.
Gebreyes WA, Jackwood D, CJB d O, Lee C-W, Hoet AE, Thakur S. Molecular epidemiology of infectious zoonotic and livestock diseases. Microbiol Spectr. United States. 2020;8 Interesting insights into the molecular epidemiology of LAMRSA.
Johler S, Stephan R, Althaus D, Ehling-Schulz M, Grunert T. High-resolution subtyping of Staphylococcus aureus strains by means of Fourier-transform infrared spectroscopy. Syst Appl Microbiol. Elsevier GmbH. 2016;39:189–94.
Fetsch, et al. Staphylococcus aureus food-poisoning outbreak associated with the consumption of ice-cream. Int J Food Microbiol. 2014;187(18):1–6.
Johler, et al. Outbreak of staphylococcal food poisoning due to SEA-producing Staphylococcus aureus. Foodborne Path. Dis. 2013;10(9):777–81.
Van den Eede A, Martens A, Feryn I, Vanderhaeghen W, Lipinska U, Gasthuys F, et al. Low MRSA prevalence in horses at farm level. BMC Vet Res. 2012;8:213.
Sahibzada S, Hernández-Jover M, Jordan D, Thomson PC, Heller J. Emergence of highly prevalent CA-MRSA ST93 as an occupational risk in people working on a pig farm in Australia. PLoS One. 2018;13:e0195510.
Butaye P, Argudín MA, Smith TC. Livestock-associated MRSA and its current evolution. Curr ClinMicrobiol Reports. 2016;3:19–31.
Walther B, Klein K-S, Barton A-K, Semmler T, Huber C, Merle R, et al. Equine Methicillin-Resistant Sequence Type 398 Staphylococcus aureus (MRSA) harbor mobile genetic elements promoting host adaptation. Front Microbiol. 2018;9:2516.
Weese JS, Archambault M, Willey BM, Hearn P, Kreiswirth BN, Said-Salim B, et al. Methicillin-resistant Staphylococcus aureus in horses and horse personnel, 2000-2002. Emerg Infect Dis. 2005;11:430–5.
Nemeghaire S, Roelandt S, Argudín MA, Haesebrouck F, Butaye P. Characterization of methicillin-resistant Staphylococcus aureus from healthy carrier chickens. Avian Pathol England. 2013;42:342–6.
Peeters LEJ, Argudín MA, Azadikhah S, Butaye P. Antimicrobial resistance and population structure of Staphylococcus aureus recovered from pigs farms. Vet Microbiol Netherlands. 2015;180:151–6.
Boss R, Cosandey A, Luini M, Artursson K, Bardiau M, Breitenwieser F, et al. Bovine Staphylococcus aureus: Subtyping, evolution, and zoonotic transfer. J Dairy Sci United States. 2016;99:515–28.
Fall C, Seck A, Richard V, Ndour M, Sembene M, Laurent F, et al. Epidemiology of Staphylococcus aureus in pigs and farmers in the largest farm in Dakar. Senegal Foodborne Pathog Dis United State. 2012;9:962–5.
Porrero MC, Wassenaar TM, Gómez-Barrero S, García M, Bárcena C, Àlvarez J, et al. Detection of methicillin-resistant Staphylococcus aureus in Iberian pigs. Lett Appl Microbiol. 2012;54(4):280–5.
Funding
Open Access funding provided by Universität Zürich.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bacteriology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fetsch, A., Etter, D. & Johler, S. Livestock-Associated Meticillin-Resistant Staphylococcus aureus—Current Situation and Impact From a One Health Perspective. Curr Clin Micro Rpt 8, 103–113 (2021). https://doi.org/10.1007/s40588-021-00170-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-021-00170-y