Abstract
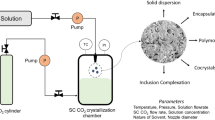
The rapid expansion of supercritical solution (RESS) process is a novel method to produce free-solvent particles with narrow particle size distribution (PSD), and it is offered to replace conventional particle formation methods. The present study is aimed to investigate the numerical simulation of the RESS process comprising hydrodynamic and particle formation and solving population balance equation to compute PSD of solute-supercritical CO2 (SC-CO2) systems. Mass, momentum and energy balances in addition to an accurate equation of state are used to calculate hydrodynamic behavior of SC-CO2 at various conditions through a nozzle and supersonic free jet with considering heat exchange in the nozzle. The solubility of TBTPP (5, 10, 15, 20-tetrakis (3, 5-bis (trifluoromethyl) phenyl) porphyrin) in SC-CO2 is calculated using the Soave–Redlich–Kwong equation of state with van der Waals mixing rule. After that, supersaturation and nucleation of TBTPP and three other drugs, i.e., aspirin, salicylic acid and ibuprofen, are investigated. Eventually, the method of moments is used to solve the population balance equations to determine PSD of the solutes. The results are presented with and without coagulation. Furthermore, the effect of nozzle length and pre-expansion temperature on PSD is studied. Furthermore, to improve the results of coagulation model on PSD, collision frequency and lognormal function are modified. The modifications improve the coagulation results. The average absolute percent deviation of TBTPP solubility is 1.86. The CO2 hydrodynamic behavior shows the same trend as reported in the literature. Moreover, results of PSD calculation with coagulation indicated there is a good agreement with the experimental ones.

















Similar content being viewed by others
References
Nelson WM (2003) Green solvents for chemistry: perspectives and practice. Oxford University Press
Bagheri H, Ghader S (2017) J Mol Liq 236:172–183
Bagheri H, Mohebbi A (2017) Korean J Chem Eng 34:2686–2702
Huang Zh, Lu WD, Kawi S, Chiew YC (2004) J Chem Eng Data 49:1323–1327
Park SJ, Kwak TY, Mansoori GA (1987) Int J Thermophys 8:449–471
Sheikhi-Kouhsar M, Bagheri H, Raeissi S (2015) Fluid Phase Equilib 395:51–57
Reverchon E, Adami R, Caputo G, Marco ID (2008) J Supercrit Fluids 47:70–84
Ye X, Wai CM (2003) J Chem Educ 80:198–204
Lotfollahi MN, Modarress H, Mansoori GA (2001) J Phys Chem B 105:9834–9839
Bagheri H, Mansoori GA, Hashemipour H (2018) J Mol Liq 261:174–188
Bagheri H, Hashemipour H, Mirzaie M (2019) J Mol Liq 281:490–505
Reverchon E, Della Porta G, Taddeo R, Pallado P, Stassi A (1995) Ind Eng Chem Res 34:4087–4091
Kwauk X, Debenedetti PG (1993) J Aerosol Sci 24:445–469
Helfgen B, Hils P, Holzknecht Ch, Türk M, Schaber K (2001) J Aerosol Sci 32:295–319
Liu J, Do-Quang M, Amberg G (2015) AIChE J 61:317–332
Yamamoto S, Furusawa T (2015) J Supercrit Fluids 97:192–201
Kwak TY, Mansoori GA (1986) Chem Eng Sci 41:1303–1309
Platzer B, Maurer G (1993) Fluid Phase Equilib 84:79–110
Hansen AG (1967) Fluid mechanics. Wiley, New York
Sandler S (1989) Chemical and engineering thermodynamics. Wiley, New York
Prausnitz JM, Lichtenthaler RN, de Azevedo EG (1998) Molecular thermodynamics of fluid-phase equilibria. Pearson Education
Reverchon E, Donsì G (1993) J Supercrit Fluids 6:241–248
Huang Zh, Sun G-B, Chiew YC, Kawi S (2005) Powder Technol 160:127–134
Sane A, Thies MC (2005) J Phys Chem B 109:19688–19695
Kayrak D, Akman U, Hortaçsu Ö (2003) J Supercrit Fluids 26:17–31
Danesh A (1998) PVT and phase behaviour of petroleum reservoir fluids, vol 47. Elsevier
Bagheri H, Ghader S, Hatami N (2019) Chem Chem Technol 13:1–10
Litster JD, Smit DJ, Hounslow MJ (1995) AIChE J 41:591–603
Lee KW, Chen H, Gieseke JA (1984) Aerosol Sci Technol 3:53–62
Pratsinis SE (1988) J Colloid Interface Sci 124:416–427
Diemer RB, Ehrman ShH (2005) Powder Technol 156:129–145
Randolph Alan D, Larson MA (1988) Theory of particulate processes-analysis and techniques of continuous crystallization. Academic Press
Salvatori F, Muhr H, Plasari E (2005) Powder Technol 157:27–32
Gruy F (2011) Colloid Surface A 374:69–76
Sane A, Taylor Sh, Sun Y-P, Thies MC (2004) J Supercrit Fluids 28:277–285
Chimowitz EH, Kelley FD, Munoz FM (1988) Fluid Phase Equilib 44:23–52
Weber M, Russell LM, Debenedetti PG (2002) J Supercrit Fluids 23:65–80
Helfgen B, Türk M, Schaber K (2000) Powder Technol 110:22–28
Reverchon E, Pallado P (1996) J Supercrit Fluids 9:216–221
Hirunsit P, Huang Z, Srinophakun T, Charoenchaitrakool M, Kawi S (2005) Powder Technol 154:83–94
Masoodiyeh F, Mozdianfard MR, Karimi-Sabet J (2017) Powder Technol 317:264–274
Flint LR, Howarths WJ (1971) Chem Eng Sci 26:1155–1168
Park SH, Lee KW, Otto E, Fissan H (1999) J Aerosol Sci 30:3–16
Sajo E (2008) Aerosol Sci Technol 42:134–139
Veerapaneni S, Wiesner MR (1996) J Colloid Interface Sci 177:45–57
Acknowledgements
The authors are grateful to professor G. A. Mansoori of the University of Illinois at Chicago to suggest useful comments and reviewing the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Soave wrote SRK EoS in the following form [26]:
where \( P_{c} \), \( T_{c} \) and \( \omega \) are critical pressure, temperature and acentric factor, respectively. Also, Eq. (36) in terms of compressibility factor would be [26]:
Equations of state are applied to multicomponent systems by using mixing rules to determine their parameters for mixtures. In this study, vdW mixing rule is used for a and b parameters [27]:
where kij is binary interaction parameters. The fugacity coefficient of a solute in a SCF is derived [26]:
Rights and permissions
About this article
Cite this article
Bagheri, H., Hashemipour, H. & Ghader, S. Population balance modeling: application in nanoparticle formation through rapid expansion of supercritical solution. Comp. Part. Mech. 6, 721–737 (2019). https://doi.org/10.1007/s40571-019-00257-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40571-019-00257-w