Abstract
Na-doped Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) materials were synthesized by a simple high-temperature solid-state method. The structure, morphology, and kinetic performances of the samples were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS). XRD indicates that all samples are in accordance with the standard LiFePO4 with olivine structure. SEM reveals that the particle size of all samples is about 1 to 2 µm. CV exhibits that Na doping obviously improves the reversibility and dynamic behaviors of lithium intercalation and deintercalation. EIS shows that Na doping decreases the charge transfer resistance of LiFePO4 and improves the lithium diffusion coefficients. It can be concluded that Na doping results in lower electrode polarization and higher lithium-ion diffusion coefficient, which can effectively improve the kinetic performance of LiFePO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries have been considered as one of the most viable candidates for EVs and HEVs because of the highest power and energy density of any existing battery systems.[1,2] Developing positive-electrode materials with high safety is one of the key challenges for lithium-ion batteries. Among the existing cathode chemistries used in lithium batteries, lithium iron phosphate (LiFePO4) offers outstanding power capability, excellent safety characteristics, and low cost compared to Ni- and Co-based layered oxide materials.[3,4] Unfortunately, LiFePO4 suffers from a very low electrical conductivity of about 10−9 s cm−1 at room temperature,[5,6] so it is difficult to achieve its theoretical capacity when discharged at a high current density. Several approaches have been explored to solve the conductivity problem. These approaches have been carried out including the reduction of particle size,[7,8] surface coatings,[9,10] or aliovalent cation doping.[11–16] The first principles’ investigation revealed that LiFePO4 is most affected by F ion doping at O site with the narrowest band gap, followed by Mn ion doping at Fe site and Na ion doping at Li site, indicating that appropriate ion doping in LiFePO4 could improve its electronic conductivity.[17,18]
Solid-state synthesis is a conventional method to prepare electrode material and exhibits a potential commercial application due to the simple synthesis route and low synthesis cost.[19,20] The chemical diffusion coefficient of lithium ion (D Li) is one of the most important kinetic characteristics of an electrochemical intercalation process in electrode materials.[21,22] The determining step of rate in the electrodes of lithium ion batteries is supposed to be the solid-state diffusion.[23] Hence, D Li plays a very important role in the study of electrodes materials for lithium-ion batteries. Many relevant techniques have been proposed for the evaluation of D Li, e.g., the capacity, potentiostatic, and galvanostatic intermittent titration technique (CITT, PITT, and GITT, respectively)[24–26] and electrochemical impedance spectroscopy (EIS).[27,28] EIS has been considered as a very powerful technology to determine the rate of individual electrode kinetic steps because it can also be obtained under more equilibrium conditions compared with other methods. In addition, the low-frequency Warburg of EIS is directly related to the lithium-ion diffusion process in an electrode material.[29]
In the paper, olivine Li1−x Na x FePO4(x = 0, 0.05, 0.1 and 0.2) is successfully synthesized by a solid-state method, and the structure and kinetic properties are evaluated.
Experimental
Analytical reagents of FeC2O4·2H2O, NH4H2PO4, Na2CO3, and Li2CO3 were used for this work. The Li1−x Na x FePO4(x = 0, 0.05, 0.1 and 0.2) composite was synthesized via a solid-state reaction. The stoichiometric proportion reagents mentioned above were placed in an agate bowl. The mixtures were ball milled in 20 mL ethanol at a rotating speed of about 200 rpm for 5 to 6 hours. After the ethanol was removed, the precursor mixture was pre-heat treated at 623 K (350 °C) for 5 hours and then 973 K (700 °C) for 10 hours under a nitrogen atmosphere. The samples were naturally cooled to room temperature.
The X-ray diffractometry (XRD) measurements were performed on a Rigaku instrument with Cu Kα radiation. Scanning electron microscopy (SEM) was performed to observe the particles morphology. Cyclic voltammetry (CV) test was carried out on a CHI-852C electrochemical workstation with a voltage between 2.5 and 4.3 V at a scanning rate of 0.1 mV/s. Electrochemical impedance spectroscopy (EIS) is measured by a CHI 760D (Shanghai Chenhua, China) electrochemical working station over a frequency range from 0.01 Hz to 10 kHz at a potentiostatic signal amplitude of 5 mV. The prepared electrode materials were adopted as the working electrode; the counter electrode and reference electrode were Li foil.
A CR2025 coin-cell assembly was used for the electrochemical characterization. Slurry was formed by mixing the Li1−x Na x FePO4 (80 pct), acetylene black (10 pct), and binder (10 wt pct polyvinylidene fluoride, dissolved in N-methyl-2-pyrrolidone). The slurry was coated onto the aluminum foil from which pellets of 14 mm in diameter were cut as electrodes. The half-cells were assembled with a composite cathode and a metallic lithium anode separated by porous polypropylene film (Celgard 2300) filled with 1 m LiPF6 in ethylene carbonate/dimethyl carbonate (1:1 by vol.) solution.
Results and Discussion
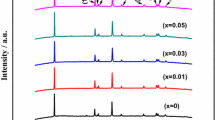
Figure 1 shows the XRD patterns of Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) samples. It can be found that the XRD patterns of the Na-doped samples are similar to that of the standard orthorhombic LiFePO4. The crystal phases of all the samples are ordered olivine structure indexed orthorhombic Pnma, and no extra reflection is observed. It is obvious that Na doping does not affect the structure of the samples, and Na+ probably enters into the lattice of LiFePO4 rather than form impurities.
Figure 2 gives the enlarged XRD patterns of the peak (101) for Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) samples. It can be found that the samples with the Na doping undergo a slight shift toward lower degrees. Since there were no peaks of impurity, and the positions of the main diffraction peaks after Na doping shift lower angle compared with the standard LiFePO4, it may be concluded that Na was inserted the internal lattice and caused an increase in interplanar distance. According to the previous report, the doping of Na ion would be inclined to occupy Li sites because the phase of NaFePO4 was structurally analogous to LiFePO4[30,31] and that Na and Li atoms have the same outer electronic structure.[32] Na+ serves as a pillar in the structure, which can stabilize the crystal structure.[32] The reason without impurity peaks may be that a partial solid solution of LiFePO4-NaFePO4 when the doped content of Na is high. The corresponding lattice parameters of Li1−x Na x FePO4 obtained from XRD are listed in Table I.
With incorporation of Na+ ions, the cell volume becomes larger. However, we note that the elementary cell volume of Li1−x Na x FePO4 is slightly less than for NaFePO4 olivine structure (V = 312.6 Å3) as the ionic volume of Na+ is almost twice that of Li+.[33] The increasing of the lattice parameter can enhance the Li+ intercalation/deintercalation.[34] This result is attributed to the larger ionic radii of Na+ (1.02 Å[35]) compared with that of Li+ (0.76 Å[35]). It has been reported that Li+ ion migration of LiFePO4 occurs preferentially via one-dimensional channels oriented along the [010] direction of the orthorhombic crystal structure due to the smallest lithium migration energy along the [010] one-dimensional tunnel with the continuous chain of edge-shearing LiO6 octahedra.[14] Hence, it can be concluded that the doping of Na can enlarge the interplanar distance of (101) crystal plane. This means that Na doping increases the Li ion pathway in [010] direction.[36]
SEM images of the pristine and doped LiFePO4 samples are shown in Figure 3. It can be seen that all samples are composed of irregular particles, and the particle sizes of the three samples are almost in the same range of 1 to 2 μm. The irregular particles may be due to the solid-state synthesis. Apparently, doping with Na+ does not change the morphology of LiFePO4. However, some agglomeration of LiFePO4 solid powders can be found. From this point of view, the particle size effect on the lithium-ion insertion kinetics of the samples may be ruled out, and the dissimilarity of the samples, in term of kinetic performance, can mainly be attributed to the Na-substitution.
Figure 4 shows the cyclic voltammograms of Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) samples measured at a sweep rate 0.1 mV s−1 in the first cycle. It is obviously that a single pair of sharp oxidation and reduction peaks, ascribing to the two-phase reaction of the Fe3+/Fe2+ redox couple, can be clearly discerned. The peaks parameters of the various samples from the CV plots are listed in Table II.
The potential differences between anodic and cathodic peaks reflect the polarization degree of the electrode. For pristine LiFePO4, the oxidation and reduction peaks occur at 3.797 and 3.182 V, respectively, with the potential difference being 615 mV. Obviously, the potential separations between the anodic and cathodic peaks for the Na-doped LiFePO4 samples are lower than those of LiFePO4. It indicates that Na doping is beneficial to the reversible intercalation and deintercalation of Li+, so it enhances the reversibility of the LiFePO4.[37] Especially, the oxidation and reduction peaks of Li0.9Na0.1FePO4 are located at 3.688 and 3.283 V, respectively, and the potential difference is 405 mV. It has been reported that a broadened peak of pristine LiFePO4 means poor kinetics, sluggish lithium intercalation, and deintercalation. However, a sharp peak of LiFePO4 highlights excellent kinetics and facile lithium intercalation and deintercalation.[38] This indicates that Na-doped LiFePO4 has lower electrochemical polarization and more excellent kinetics than that of pristine LiFePO4. The lower polarization and excellent kinetics of Na-doped LiFePO4 samples can be attributed to the higher electronic conductivity improved by Na doping compared with that of LiFePO4.
In order to further study the effect of Na doping on the kinetics process of the electrode materials, electrochemical impedance spectroscopy (EIS) measurement of Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) was carried out in the frequency range between 10 kHz and 0.1 Hz, which is shown in Figure 5. The inset shows the selected equivalent circuit used to fit the EIS and the enlarged Nyquist plots of the Li1−x Na x FePO4 materials for x = 0.05 and x = 0.1. All curves show one compressed semicircle in the medium-frequency region and an inclined line in the low-frequency range.
The high-frequency semicircle always corresponds to the charge transfer process (R ct),[39,40] and the fitted R ct data are listed in Table II. It was found the overall charge transfer resistance of the Na-doped electrodes decreased gradually with the increase of Na concentration (x ≤ 0.1), and it was much smaller than that of the pristine LiFePO4. Li0.9Na0.1FePO4 exhibits the smallest charge transfer resistance among all samples, suggesting that Li0.9Na0.1FePO4 may have the highest electrochemical activity during cycling. The R s reflects electric conductivity of the electrolyte, separator, and electrodes, and the fitted R s data are listed in Table II.
It can be found that the R s values of the Na-doped electrodes are much smaller than that of the pristine LiFePO4, indicating that Na-doping electrodes exhibit much smaller solution than the pristine LiFePO4. In the low-frequency region, the sloping line of Na-doped LiFePO4 is larger than pristine LiFePO4, revealing that the Na-doped LiFePO4 are more favorable for the intercalation/deintercalation of lithium ions into/from the bulk solid phase.[41] This indicates that Na doping can enhance the electronic conductivity of LiFePO4. The straight line with a slope in low-frequency region is attributed to the diffusion of the lithium ions into the bulk of the electrode material or the so-called Warburg diffusion.[42,43] The lithium-ion diffusion coefficient (D Li) can be calculated from the plots in the low-frequency region according to the following equations (Eqs. [1] and [2])[44–52]—similar approach has also been applied in various cathode materials like LiFePO4,[46–48] LiNi0.5Mn1.5O4,[49] LiV3O8,[50] LiMn2SiO4,[51] and Li4Ti5O12[52] electrode materials:
where T is the absolute temperature; R is the gas constant; A is the surface area of the electrode; F is the Faraday constant; n is the number of electrons per molecule during oxidation; C Li is the concentration of lithium ion; and σ is the Warburg factor which is related to Z re obtained from the slope of the lines in Figure 6. The selected low-frequency region ranges from 0.01 to 0.1 Hz.
The diffusion coefficient values of Li1−x Na x FePO4 (x = 0, 0.05, 0.1 and 0.2) are 1.21 × 10−16, 1.06 × 10−14, 7.95 × 10−15, and 9.17 × 10−15 cm2 s−1, respectively. It is obvious that the lithium diffusion coefficients increase due to the Na doping. This result clearly indicates that the lithium-ion mobility of LiFePO4 can be effectively improved by moderate Na doping. The reason maybe is that the Na doping increases the Li ion pathway in [010] direction.
Conclusions
Li1−x Na x FePO4 (x = 0, 0.05, 0.1, and 0.2) powders were successfully synthesized by a simple high-temperature solid-state method. Na ions have successfully been doped into the LiFePO4 phase, and the particle size of all samples is around 1 to 2 µm. The low potential interval demonstrates that the lithium insertions into the Na-doped LiFePO4 electrodes can be treated as a quasi-reversible system with improved dynamic behaviors. Na doping can not only reduce the charge transfer resistance but also increase the diffusion coefficient of lithium ion. Therefore, the Na doping is an effective approach to achieve excellent kinetic performance for the LiFePO4 positive-electrode materials.
References
J. Yang, X.-Y. Zhou, Y.-L. Zou, J.-J. Tang, Electrochim. Acta 56, 8576–81 (2011)
M. Masih-Tehrani, M.-R. Ha’iri-Yazdi, V. Esfahanian, A. Safaei, J. Power Sour. 244, 2–10 (2013)
L. Dimesso, C. Förster, W. Jaegermann, J.P. Khanderi, H. Tempel, A. Popp, J. Engstler, J.J. Schneider, A. Sarapulova, D. Mikhailova, L.A. Schmitt, S. Oswald, H. Ehrenberg, Chem. Soc. Rev. 41, 5068–5080 (2012)
N. Alias, A.A. Mohamad, J. Power Sour. 274, 237–251 (2015)
J. Lee, P. Kumar, J. Lee, B.M. Moudgil, R.K. Singh, J. Alloys Compd. 550, 536–544 (2013)
H.-J. Kim, J.-M. Kim, W.-S. Kim, H.-J. Koo, D.-S. Bae, H.-S. Kim, J. Alloys Compd. 509, 5662–5666 (2011)
J. Mun, H.-W. Ha, W. Choi, J. Power Sour. 251, 386–392 (2014)
E. Hosono, Y. Wang, N. Kida, M. Enomoto, N. Kojima, M. Okubo, H. Matsuda, Y. Saito, T. Kudo, I. Honma, H. Zhou, ACS Appl. Mater. Interfaces 2, 212–218 (2010)
T. Kozawa, N. Kataoka, A. Kondo, E. Nakamura, H. Abe, M. Naito, Ceram. Int. 40, 16127–16131 (2014)
D. Ziolkowska, K.P. Korona, B. Hamankiewicz, S.-H. Wu, M.-S. Chen, J.B. Jasinski, M. Kaminska, A. Czerwinski, Electrochim. Acta 108, 532–539 (2013)
R. Trócoli, S. Franger, M. Cruz, J. Morales, J. Santos-Peña, Electrochim. Acta 135, 558–567 (2014)
A.Y. Shenouda, H.K. Liu, J. Alloys Compd. 477, 498–503 (2009)
K.L. Harrison, C.A. Bridges, M.P. Paranthaman, C.U. Segre, J. Katsoudas, V.A. Maroni, J.C. Idrobo, J.B. Goodenough, A. Manthiram, Chem. Mater. 25, 768–781 (2013)
M.S. Islam, D.J. Driscoll, C.A.J. Fisher, P.R. Slater, Chem. Mater. 17, 5085–5092 (2005)
T. Honma, K. Nagamine, T. Komatsu, Ceram. Int. 36, 1137–1141 (2010)
I. Bilecka, A. Hintennach, M.D. Rossell, D. Xie, P. Novák, M. Niederberger, J. Mater. Chem. 21, 5881–5890 (2011)
J. Xu, G. Chen, Physica B 405, 803–807 (2010)
M. Milović, D. Jugović, N. Cvjetićanin, D. Uskoković, A.S. Milošević, Z.S. Popović, F.R. Vukajlović, J. Power Sour. 241, 70–79 (2013)
D. Jugović, D. Uskoković, J. Power Sour. 190, 538–544 (2009)
J.W. Fergus, J. Power Sour. 195, 939–954 (2010)
F. Lantelme, A. Mantoux, H. Groult, D. Lincot, Solid State Ionics 177, 205–209 (2006)
H. Manjunatha, T.V. Venkatesha, G.S. Suresh, Electrochim. Acta 58, 247–257 (2011)
X.H. Rui, N. Ding, J. Liu, C. Li, C.H. Chen, Electrochim. Acta 55, 2384–2390 (2010)
H. Manjunatha, K.C. Mahesh, G.S. Suresh, T.V. Venkatesha, Electrochim. Acta 80, 269–281 (2012)
C. Montella, Electrochim. Acta 51, 2778–2781 (2006)
D.W. Dees, S. Kawauchi, D.P. Abraham, J. Prakash, J. Power Sour. 189, 263–268 (2009)
M. Talebi-Esfandarani, O. Savadogo, Solid State Ionics 261, 81–86 (2014)
K. Tang, X. Yu, J. Sun, H. Li, X. Huang, Electrochim. Acta 56, 4869–4875 (2011)
T.-F. Yi, Y. Xie, Q. Wu, H. Liu, L. Jiang, M. Ye, R. Zhu, J. Power Sour. 214, 220–226 (2012)
J.N. Birdson, S.E. Quinlan, P.R. Tremaine, Chem. Mater. 10, 763–768 (1998)
V. Palomares, P. Serras, I. Villaluenga, K.B. Hueso, J. Carretero-González, T. Rojo, Energy Environ. Sci. 5, 5884–5901 (2012)
Z.H. Wang, L.X. Yuan, M. Wu, D. Sun, Y.H. Huang, Electrochim. Acta 56, 8477–8483 (2011)
K. Zaghib, J. Trottier, P. Hovington, F. Brochu, A. Guerfi, A. Mauger, C.M. Julien, J. Power Sour. 196, 9612–9617 (2011)
Z. Ma, G. Shao, G. Wang, Y. Zhang, J. Du, J. Solid State Chem. 210, 232–237 (2014)
R.D. Shannon, Acta Crystallogr. A3, 751–767 (1976)
D. Morgan, A.V. der Ven, G. Ceder, Electrochem. Solid State Lett. 7, A30–A32 (2004)
C. Gong, Z. Xue, X. Wang, X.-P. Zhou, X.-L. Xie, Y.-W. Mai, J. Power Sour. 246, 260–268 (2014)
G. Qin, Q. Wu, J. Zhao, Q. Ma, C. Wang, J. Power Sour. 248, 588–595 (2014)
M.M. Rahman, J.-Z. Wang, R. Zeng, D. Wexler, H.K. Liu, J. Power Sour. 206, 259–266 (2012)
K. Bazzi, B.P. Mandal, M. Nazri, V.M. Naik, V.K. Garg, A.C. Oliveira, P.P. Vaishnava, G.A. Nazri, R. Naik, J. Power Sour. 265, 67–74 (2014)
C. Miao, P. Bai, Q. Jiang, S. Sun, X. Wang, J. Power Sour. 246, 232–238 (2014)
O. Toprakci, H.A.K. Toprakci, L. Ji, G. Xu, Z. Lin, X. Zhang, ACS Appl. Mater. Interfaces 4, 1273–1280 (2012)
L. Wang, Z. Li, H. Xu, K. Zhang, J. Phys. Chem. C 112, 308–312 (2008)
M. Vujković, I. Stojković, N. Cvjetićanin, S. Mentus, Electrochim. Acta 92, 248–256 (2013)
N. Takami, A. Satoh, M. Hara, T. Ohsaki, J. Electrochem. Soc. 142, 371–379 (1995)
H. Liu, Q. Cao, L.J. Fu, C. Li, Y.P. Wu, H.Q. Wu, Electrochem. Commun. 8, 1553–1557 (2006)
X. Qin, X. Wang, J. Xie, L. Wen, J. Mater. Chem. 21, 12444–12448 (2011)
Y. Zhou, J. Wang, Y. Hu, R. O’Hayre, Z. Shao, Chem. Commun. 46, 7151–7153 (2010)
J. Wang, W. Lin, B. Wu, J. Zhao, Electrochim. Acta 145, 245–253 (2014)
S. Sarkar, H. Banda, S. Mitra, Electrochim. Acta 99, 242–252 (2013)
S. Liu, J. Xu, D. Li, Y. Hu, X. Liu, K. Xie, J. Power Sour. 232, 258–263 (2013)
B. Li, C. Han, Y.B. He, C. Yang, H. Du, Q.H. Yang, F. Kang, Energy Environ. Sci. 5, 9595–9602 (2012)
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 51404002, 51274002), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Dr. J. Yao), National Undergraduate Training Programs for Innovation and Entrepreneurship (201410360022), Anhui Undergraduate Training Programs for Innovation and Entrepreneurship, and the Program for Innovative Research Team in Anhui University of Technology (No. TD201202).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Manuscript submitted September 2, 2014.
Rights and permissions
About this article
Cite this article
Zhu, YR., Zhang, R., Deng, L. et al. Lithium-Ion Insertion Kinetics of Na-Doped LiFePO4 as Cathode Materials for Lithium-Ion Batteries. Metallurgical and Materials Transactions E 2, 33–38 (2015). https://doi.org/10.1007/s40553-014-0041-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40553-014-0041-6