Abstract
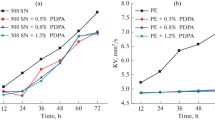
An oil soluble multifunctional protic ionic liquid (IL) was synthesized and its tribological and antioxidant properties in poly alpha olefin (PAO4) were investigated. The tribological results demonstrated that the IL significantly reduced friction and wear of PAO4. The PAO4 blend with IL resulted in an induced oxidation time of 555 min which is 8.2 and 3.5 times higher than that of pure PAO4 and PAO4 with zinc dialkyl dithiophosphate (ZDDP) for the rotating pressure vessel oxidation test. It is likely that free nonylated diphenylamine acted as a radical scavenger to enhance antioxidant performance, while free bis(2-ethylhexyl) phosphate was more prone to adsorb and react with the metal surface to form a phosphorus-rich tribofilm in order to protect the rubbing surface.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Earle M J, Seddon K R. Ionic liquids. Green solvents for the future. Pure Appl Chem72(7): 1391–1398 (2000)
Ye C, Liu W, Chen Y, Yu L. Room-temperature ionic liquids: A novel versatile lubricant. Chem Commun21: 2244–2245 (2001)
Patel D D, Lee J-M. Applications of ionic liquids. Chem Rec12(3): 329–355 (2012)
Plechkova N V, Seddon K R. Applications of ionic liquids in the chemical industry. Chem Soc Rev37(1): 123–150 (2008)
Jiménez A E, Bermúdez M D, Carrión F J, Martínez-Nicolás G. Room temperature ionic liquids as lubricant additives in steel-aluminium contacts: Influence of sliding velocity, normal load and temperature. Wear261(3–4): 347–359 (2006)
Jiménez A E, Bermúdez M D, Iglesias P, Carrión F J, Martínez-Nicolás G. 1-n-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel-aluminium contacts. Wear260(7–8): 766–782 (2006)
Kamimura H, Kubo T, Minami I, Mori S. Effect and mechanism of additives for ionic liquids as new lubricants. Tribology International40(4): 620–625 (2007)
Xie G, Wang Q, Si L, Liu S, Li G. Tribological characterization of several silicon-based materials under ionic-liquids lubrication. Tribol Lett36(3): 247–257 (2009)
Minami I, Inada T, Sasaki R, Nanao H. Tribo-chemistry of phosphonium-derived ionic liquids. Tribol Lett40(2): 225–235 (2010)
Jin C-M, Ye C, Phillips B S, Zabinski J S, Liu X, Liu W, Shreeve JM. Polyethylene glycol functionalized dicationic ionic liquids with alkyl or polyfluoroalkyl substituents as high temperature lubricants. J Mater Chem16(16): 1529–1535 (2006)
Jiang C, Li W, Nian J, Lou W, Wang X. Tribological evaluation of environmentally friendly ionic liquids derived from renewable biomaterials. Friction6(2): 208–218 (2018)
Yu B, Bansal D G, Qu J, Sun X, Luo H, Dai S, Blau P J, Bunting B G, Mordukhovich G, Smolenski D J. Oil-miscible and non-corrosive phosphonium-based ionic liquids as candidate lubricant additives. Wear289: 58–64 (2012)
Barnhill W C, Qu J, Luo H, Meyer H M, Ma C, Chi M, Papke B L. Phosphonium-organophosphate ionic liquids as lubricant additives: Effects of cation structure on physicochemical and tribological characteristics. ACS Appl Mater Inter6(24): 22585–22593 (2014)
Elsentriecy H H, Qu J, Luo H, Meyer H M, Ma C, Chi M. Improving corrosion resistance of AZ31B magnesium alloy via a conversion coating produced by a protic ammoniumphosphate ionic liquid. Thin Solid Films568: 441–51 (2014)
Fan M, Liang Y, Zhou F, Liu W. Dramatically improved friction reduction and wear resistance by in situ formed ionic liquids. RSC Adv2(17): 6824–6830 (2012)
Fan M, Song Z, Liang Y, Zhou F, Liu W. In situ formed ionic liquids in synthetic esters for significantly improved lubrication. ACS Appl Mater Inter4(12): 6683–6689 (2012)
Song Z, Liang Y, Fan M, Zhou F, Liu W. Lithium-based ionic liquids as novel lubricant additives for multiply alkylated cyclopentanes (MACs). Friction1(3): 222–231 (2013)
Cai M, Liang Y, Zhou F, Liu W. Functional ionic gels formed by supramolecular assembly of a novel low molecular weight anticorrosive/antioxidative gelator. J Mater Chem21(35): 13399–13405 (2011)
Cai M, Liang Y, Zhou F, Liu W. Anticorrosion imidazolium ionic liquids as the additive in poly(ethylene glycol) for steel/Cu-Sn alloy contacts. Faraday Discuss156(1): 147–157 (2012)
Cai M, Liang Y, Zhou F, Liu W. A novel imidazolium salt with antioxidation and anticorrosion dual functionalities as the additive in poly(ethylene glycol) for steel/steel contacts. Wear306(1–2): 197–208 (2013)
Zhang Y, Zeng X, Wu H, Li Z, Ren T, Zhao Y. The tribological chemistry of a novel borate ester additive and its interaction with ZDDP using XANES and XPS. Tribol Lett53(3): 533–542 (2014)
Rizvi S Q A. A comprehensive review of lubricant chemistry, technology, selection, and design. ASTM International, USA, 2009
Chao M, Li W, Chen L, Wang X. Hindered phenol derivative as a multifunctional additive in lithium complex grease. Ind Eng Chem Res54(26): 6605–6610 (2015)
Qu J, Barnhill W C, Luo H, Meyer H M, Leonard D N, Landauer A K, Kheireddin B, Gao H, Papke B L, Dai S. Synergistic effects between phosphonium-alkylphosphate ionic liquids and zinc dialkyldithiophosphate (ZDDP) as lubricant additives. Adv Mater27(32): 4767–4774 (2015)
Zhan W, Tu J S, Qian X Z, Li J, Liu J. Synthesis of butyloctyl-diphenylamine as lubricant antioxidant additive by ionic liquids. Int J Adv Manuf Technol96(5): 1647–1653 (2018)
Qu J, Luo H M, Chi M F, Ma C, Blau P J, Dai S, Viola M B. Comparison of an oil-miscible ionic liquid and ZDDP as a lubricant anti-wear additive. Tribology International71: 88–97 (2014)
Zhou Y, Qu J. Ionic liquids as lubricant additives: A review. ACS Appl Mater Inter9(4): 3209–3222 (2017)
Ratoi M, Niste V B, Alghawel H, Suen Y F, Nelson K. The impact of organic friction modifiers on engine oil tribofilms. RSC Adv4(9): 4278–4285 (2014)
Spikes H. The history and mechanisms of ZDDP. Tribol Lett17(3): 469–489 (2004)
Barnes A M, Bartle K D, Thibon V R A. A review of zinc dialkyldithiophosphates (ZDDPs): Characterisation and role in the lubricating oil. Tribology International34(6): 389–395 (2001)
Topolovec-Miklozic K, Forbus T R, Spikes H A. Film thickness and roughness of ZDDP antiwear films. Tribol Lett26(2): 161–171 (2007)
Taylor L J, Spikes H A. Friction-enhancing properties of ZDDP antiwear additive: Part I—friction and morphology of ZDDP reaction films. Tribol T46(3): 303–309 (2003)
Taylor L J, Spikes H A. Friction-enhancing properties of ZDDP antiwear additive: Part II—influence of ZDDP reaction films on EHD lubrication. Tribol T46(3): 310–314 (2003)
Kawada S, Watanabe S, Tadokoro C, Tsuboi R, Sasaki S. Lubricating mechanism of cyano-based ionic liquids on nascent steel surface. Tribology International119: 474–480 (2018)
Singh A, Gandra R T, Schneider E W, Biswas S K. Studies on the aging characteristics of base oil with amine based antioxidant in steel-on-steel lubricated sliding. J Phys Chem C117(4): 1735–1747 (2013)
Greaves T L, Drummond C J. Protic ionic liquids: Properties and applications. Chem Rev108(1): 206–237 (2007)
Fumino K, Wulf A, Ludwig R. The potential role of hydrogen bonding in aprotic and protic ionic liquids. Phys Chem Chem Phys11(39): 8790–8794 (2009)
Earle M J, Esperança J M S S, Gilea M A, Canongia Lopes J N, Rebelo L P N, Magee J W, Seddon K R, Widegren J A. The distillation and volatility of ionic liquids. Nature439: 831 (2006).
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant Nos. 51605471, 51505460 and 51775536).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cheng JIANG. She received her Ph.D. degree in Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences in 2013. She is an assistant research fellow in State Key Laboratory of Solid Lubrication at Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences. Her research interests mainly focus on high performance lubricants.
Wenjing LOU. She received her Ph.D. degree in Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences in 2007. She is an associate researcher in the State Key Laboratory of Solid Lubrication at Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences. Her research areas include nanofluids, nanoparticle lubricant additives, and high performance lubricants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article′s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article′s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, C., Wang, Y., Su, H. et al. Synthesis and evaluation of a protic ionic liquid as a multifunctional lubricant additive. Friction 8, 568–576 (2020). https://doi.org/10.1007/s40544-019-0283-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40544-019-0283-5