Abstract
Purpose of review
Fish is a common elicitor of IgE-mediated food allergy. Fish includes a large variety of foods, in terms of species and food processing, with marked distinction in local diets around the globe. Fish-allergic patients present with phenotypic diversity and major differences in levels of clinical cross-reactivity, features that pose an important challenge for the clinical diagnosis and management.
Recent findings
Parvalbumin is the major fish allergen. However, a single molecule is not sufficient but several homologs, allergens different from parvalbumin and allergen extracts, are needed for IgE-based diagnosis.
Summary
Parvalbumin-specific IgE are markers for clinical cross-reactions. Added value is provided by IgE typing to parvalbumin homologs from distantly related fish. IgE co-sensitization profiles (parvalbumin, enolase, aldolase) are referred as severity markers. The allergen panel seems to be not yet complete why fish extracts still play a crucial role in serum IgE analysis. Further clinical validation of a multiplex approach in molecular fish allergy diagnosis is needed for striving to avoid unnecessary food restrictions and in a further sense, improved patient care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish is a diverse food that is popular in many human diets around the globe and beyond, it is considered to be a healthy alternative to meat. The ingestion of fish or contact with fish can be a source for adverse reactions while IgE-mediated allergies are considered the most common type. In addition to milk, eggs, peanuts, tree nuts, soy, wheat and seafood, fish is counted among the most frequent triggers of IgE-mediated food allergies [1]. Fish is also an important cause of occupational allergies [2, 3]. Clinical symptoms involve single or several organs, ranging from mild to severe anaphylaxis. As an animal food source, fish is highly diverse exhibiting the largest species diversity among vertebrates. Allergenic molecules vary in different fishes. This, along with various fish preparation methods causing allergen modifications, causes human exposure to a broad variety of intact and modified allergens. Fish-allergic patients are characterized by phenotypic diversity, with major differences in levels of clinical cross-reactivity (e.g. fish-fish). This article will provide an overview about the current state-of-knowledge around IgE-mediated fish allergy and the most important fish allergens. Diagnostic challenges related to the molecular component diagnostic approach will be discussed in the light of unmet medical needs.
Fish: healthy variety on the plate
Fish is an important supplier of protective omega-3 fatty acids, high levels of protein, various trace elements and lipid-soluble vitamins. A total of over 700 fish species are available commercially, most of the species are bony fishes (Osteichthyes) [4]. Frequently consumed edible fish belong to the following families: salmons (e.g. Atlantic/Pacific salmon, trout), cod-like fish (e.g. Atlantic cod, Alaska pollock), flatfishes (e.g. plaice, sole), perch-like fish (e.g. tuna, mackerel, swordfish), herring-like fish (e.g. herring, sardine, anchovy), carp-like fish (e.g. carp, barbel) and catfish-like (e.g. catfish, pangasius) [5••]. Bony fish have mostly light muscle tissue; typical dark muscle fishes are pelagic species like tuna, herring and mackerel (www.fao.org). The light muscle is adapted to rapid movements, the dark muscle to continuous long-range swimming. Cartilaginous fish (Chondrichthyes), rays and sharks are distant relatives of bony fish [6•]. While bony fish satisfy the global market, the consumption of cartilaginous fish is limited to specific regions. According to estimates by the Food and Agriculture Organization of the United Nations (FAO), there is a growing demand worldwide for fish and fishery products. The consumption of fish varies greatly over the world, depending on eating habits and local supply (e.g. ca. 22 kg/capita/year in Europe or Northern America; ca. 42 kg/capita/year in China). Consumed fish species vary in different geographical regions. Cod, salmon, tuna and Alaska pollock are among the top species in Europe while in Asia-Pacific regions, others are popular (e.g. tilapia, catfish, perch and snakehead) (www.fao.org). Furthermore, preparation methods vary widely, from raw over to strongly processed fish. Variable global patterns of fish consumption (e.g. species, processing) entail a wide spectrum of fish antigens in human exposure.
Beyond IgE-mediated fish allergy
Different types of adverse reactions to fish are known, immunological and non-immunological reactions. Briefly, the following categories need to be distinguished from the classical type of genuine type I hypersensitivity.
Food protein-induced enterocolitis syndrome
This cellular type of food allergy manifests with delayed (1–4 h) gastrointestinal symptoms after ingestion [7]. Among others (e.g. cow’s milk, soy, rice), fish belongs to the most prevalent foods triggering food protein-induced enterocolitis syndrome (FPIES), especially in the Mediterranean area [8, 9]. The clinical diagnosis is based on a detailed medical history (e.g. clinical symptoms, timing). No laboratory tests are available to confirm fish-related FPIES diagnosis.
Fish allergy-like symptoms by toxins
Specific fish, such as tuna or mackerel (scombroids), contain high muscle levels of histidine. Upon bacterial fish spoiling, histidine decomposition results in the histamine formation [10]. Histamine might be also formed during fish processing such as canning. The intake of the histamine-rich fish induces allergy-like symptoms [11]. The diagnostic work-up relies on the clinical history including absence of specific IgE and low reproducibility of the adverse reaction.
Anisakis-induced symptoms
Anisakis is a parasitic worm infecting fish muscle. Anisakis-spoiled fish, such as in sushi, can induce human illness (anisakiasis) with inflammatory intestinal symptoms [12, 13]. Anisakis is also causing IgE-mediated allergy to helminth allergens, mostly excretion/secretion parasite molecules [14]. It has been reported that only the ingestion of life parasites leads to clinical symptoms [15]. The medical diagnosis of Anisakis-induced adverse reaction is based on the anamnesis (clinical manifestation, fish preparation, reaction reproducibility), as well as in the case of type I food allergy, serum IgE testing with Anisakis extract.
IgE-mediated fish allergy in review
Epidemiology
Fish is one of the most common triggers of IgE-mediated food allergies. Questionnaire-based studies revealed prevalence rates of 0 to 7% (e.g. USA 0.2%, Greece 1.5%, Finland 7%). Allergic sensitization (skin, serum) identified up to 2.9% of the individuals (e.g. China 0.2%, Norway 1.1%, Germany 2.9%). Finally, food challenge-confirmed prevalence rates range up to 0.3% (e.g. Denmark 0.2%, Iceland 0.3%) [16]. The prevalence of fish allergy is higher in regions with frequent fish exposure (diet, processing industries). Of note, prevalence datasets provide an informative basis but are specific for the underlying study conditions (e.g. participants’ age, fish species in food challenge) [17, 18].
Pathogenesis
Early events play a critical role for immune development, both tolerance development and breakdown [19]. Whether early introduction (before 12 months of age) does contribute to prevent fish allergy still needs further investigation [20]. Including fish into the diet of young infants seems to reduce the risk for developing asthma and allergic rhinitis [21, 22]. Many patients develop clinical fish allergy during childhood that persist during adulthood [16]. Adulthood onset of fish allergy, both as classical food allergy and occupational allergy, is also known. Clinical symptoms occur within minutes after fish ingestion, inhalation and skin contact, respectively, leading to cutaneous (urticaria, angioedema), gastrointestinal (oral allergy syndrome, laryngeal edema, spasm, diarrhea, vomiting) and respiratory manifestation (rhino conjunctivitis, bronchospasm) as well as in some cases, severe anaphylaxis [3, 23••, 24].
Clinical reactivity profiles
Patients react primarily to fish that are part of their diet. Still, those individuals have a considerable risk of developing adverse symptoms with several, if not all, species of fish. Clinical cross-reactivity was considered long time as a hallmark of fish allergy. Studies of the recent decade revealed more differentiated insights into reactivity profiles.
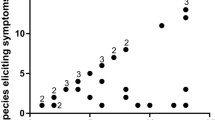
Food challenge-based studies on fish cross-reactivity are rare. Previous studies reported on high levels of cross-reactivity related to IgE testing and clinical history [25, 26]. Several studies recorded data based on questionnaires. A Dutch study (total, n = 38) reported that 59% of the fish-allergic patients had an allergy to all fish species ever tried [27]. A Japanese study (total, n = 38) referred high cross-reactivity in 88% of the participants [28]. A recent study (total, n = 35) analyzed clinical cross-reactivity in a double-blind placebo-controlled food challenge (DBPCFC) design [23••]. According to objective symptom scoring, 43% of the participants reacted to all studied fishes while 54% tolerated at least one fish (subjective symptoms scoring: 68% non-tolerant, 29% partially tolerant). Thus, the overall prevalence of patients with broad and limited cross-reactivity might be estimated at 70% and 30%, respectively (Fig. 1). Another study (total, n = 18) revealed cross-reactivity among bony fishes and in most patients, tolerance to ray [29•]. Food allergy to a single fish has been reported in case studies (e.g. cod, salmon, sole, swordfish, catfish and conger fish) [30,31,32,33,34,35] and limited cross-reactivity to tuna/marlin and pangasius/tilapia [36, 37]. The percentage of patients with monoreactivity to salmonids (salmon, trout) was 12% in another European cohort (total, n = 62) [38].
Allergic sensitization to fish is primed by dietary conditions (species, processing). Patients feature broad to limited cross-reactivity in 70% and 30%, respectively, of the cases. Parvalbumins are important cross-reactivity markers, with higher IgE-titers in patients of broad as compared with those of low cross-reactivity.
Medical diagnosis
As for other food allergies, the DBPCFC is the golden standard in fish allergy diagnosis. Low symptoms-eliciting doses have been reported (e.g. cod ED10, 0.7 mg and 23.8 mg for subjective and objective symptoms, respectively) [23••, 39]. Food challenges are usually not performed as the testing is extensive and with inherent health risk for the patient. Diagnostic mainstays are the careful record of the clinical history and IgE tests. Direct IgE tests include serum titration using fish extracts and optionally, fish parvalbumin from carp or cod (Table 1). Skin reactivity testing using fish or commercial fish extracts can be done in addition. Upon established diagnosis, the clinical management relies on a strict avoidance diet and medication of adverse symptoms [40].
Fish is one of the food allergens that need to be labeled mandatorily on all products irrespective of the percentage in the food [41]. This legislation has been implemented in order to contribute to the better safety of allergic patients. Still, this does not prevent accidental fish intake.
Occupational fish allergy
The occupational environment of a fish-processing working place entails important levels of exposure to allergens [42, 43]. A great load with airborne fish proteins (up to 986 ng.m-3) including allergens has been measured for the fish-processing environment [44]. Cooks have high exposure rates by skin contact, inhalation but also ingestion [3]. Cutaneous symptoms (contact urticaria, protein contact dermatitis), allergic rhinitis and asthma are the most common symptoms. Preceding atopy and hand eczema are risk factors for fish sensitization via the damaged skin barrier [45]. The current diagnostic work-up includes sera IgE testing and skin tests (prick, prick-to-prick) with diagnostic fish extracts or the native food [3].
Fish allergens
Parvalbumins
The major fish allergen is parvalbumin [46]. This acidic muscle protein (10–12 kDa) occurs in all fish even though molecular characteristics vary across the species. Parvalbumin belongs to the family of divalent ion-binding ‘EF-hand’ proteins. They are involved in cellular ion homeostasis and muscle relaxation. Fish parvalbumin has two functional motifs (‘EF-hand motifs’) binding calcium- or magnesium-ions. The apo-protein has a lower IgE-binding capacity as compared with the ion-charged molecule, concluding that important IgE epitopes are located in the ion-binding regions [47]. Parvalbumin mutants with modified EF-hand motifs feature reduced IgE-binding capacity and low stability to gastrointestinal digestion [48, 49]. Otherwise, parvalbumin has great molecular stability under thermal, chemical and proteolytic conditions.
Parvalbumins are clustered into the α- and the β-lineage [6, 46]. The β-lineage has a lower isoelectric point (pI < 4.8) compared with the α-lineage. Bony fish muscle contains mostly β-parvalbumin while α-parvalbumin is expressed by cartilaginous fish (e.g. rays and sharks). Most bony fish express several allergenic parvalbumins. The allergome database comprises 257 allergenic parvalbumins from 230 fish species (www.allergome.org accessed on 2019-07-16). Parvalbumins approved by the Allergen Nomenclature World Health Organization (WHO)/International Union of Immunological Societies (IUIS) Sub-Committee are summarized in Table 1.
Various studies showed high IgE prevalence to parvalbumins (> 70%) in fish-allergic patients, such as in European patients, as well as patients with reactivity to Asia-Pacific fish species [23••, 38, 50, 51]. Cross-reactive parvalbumin B cell epitopes are located in highly conserved protein regions, especially at the ion-binding sites [47]. Also, molecule-specific epitope regions are known, such as for salmonid fish parvalbumins, that explain limited cross-reactivity to those species [30, 31]. IgE cross-inhibition testing may reveal the patient’s primary sensitization by determination of the most potent inhibitor protein [29•, 38]. Levels of IgE cross-reactivity vary, with high cross-recognition among parvalbumins from closely related fish and low cross-recognition in distantly related fish [52, 53]. That way, low IgE cross-recognition of ray α-parvalbumin was found in patients with primary sensitization to the bony fish homologue β-parvalbumin [29•]. This correlated with the patients’ clinical reactivity as most tolerated ray. A subgroup of parvalbumin-positive patients has IgE antibodies recognizing not only fish β-allergen but also α-homologs from different meats (frog, chicken, crocodile) (Table 2) [53,54,55,56]. Unexpected clinical cross-reactions are known to occur in those patients.
Parvalbumin is found in less amount in dark fish muscle whereas light fish muscle contains high levels. Fish with light muscle tissue, such as cod or carp, have a high parvalbumin content of ca. 2.5–5.0 mg allergen/g muscle tissue while the allergen is mostly not detectable in dark muscle fish (e.g. tuna and swordfish) [57, 58]. Insights on parvalbumin contents are of translational relevance [59]. Low parvalbumin content fish are often tolerated, essentially in those patients with parvalbumin-specific sensitization only.
Food processes like heating and canning can result in parvalbumin epitope modification and thus, finally affecting IgE recognition [60, 61]. Parvalbumins can oligomerize or even form high molecular weight states, entailing also important changes in IgE binding [62]. Overall, fish canning seems to reduce IgE binding.
Enolases and aldolases
These muscle proteins are minor fish allergens. Both glycolytic enzymes occur in different isoforms. They belong to the family of ‘TIM barrel proteins’ characterized by a conserved tertiary protein folding of a barrel-like structure. Enolase binds magnesium-ions. Enolase (50 kDa) and aldolase (40 kDa) were identified as fish allergens in cod, salmon and tuna (Table 1) but also blunt snout bream and Nile perch [38, 63,64,65]. The prevalence of IgE binding seems to vary greatly for different fishes. In a previous study, 56% of the patients had IgE antibodies to cod enolase but only 19% to tuna enolase, 37% to cod aldolase but only 13% IgE to tuna aldolase [38]. Higher prevalence of cod-specific IgE in comparison with salmon and mackerel homologues was confirmed recently [23••]. Co-sensitization to cod parvalbumin, enolase and aldolase was concluded to correlate to severity of the clinical reaction to this fish [23••]. Both fish enolase and aldolase seem to be cross-reactive (with high inter-individual variation), although to a lower extent as compared with parvalbumins. Both enolase and aldolase are labile to thermal treatment [29•, 38].
Collagen and gelatin
Collagen and gelatin are minor fish allergens in European patients. Fish collagen is a highly stable protein mainly found in the skin, bone and other connective tissue [66]. This large molecule (300–400 kDa) is composed of three, helix-twisted chains. Glycine, alanine, proline and hydroxyproline are the major amino acids present in the primary structure. Gelatin is derived from fibrous collagen, by acidic or alkaline treatment, resulting in a water-soluble peptide mix.
First, IgE reactivity to tuna collagen was detected in fish-allergic patients from Japan [67]. Severe anaphylaxis was reported also in a clinical case with fish gelatin hidden in sweets [68]. The IgE prevalence for fish gelatin was found 19.3% in 62 fish-allergic individuals from Europe [38]. Later, IgE reactivity against mackerel collagen was confirmed for Japanese patients (50%; total, n = 34), including demonstration of effector cell reactivity triggered by the food allergen and cross-reactivity between homologs from 22 different fish species. IgE cross-reactivity appears to be limited for collagens from bony and cartilaginous fish [69,70,71]. So far, two fish collagens have been approved as official allergens, homologues from Atlantic salmon (Sal s 6) and barramundi (Lat c 6) (Table 1). Fish collagen has to be declared on food products in the USA but in Europe, it is exempted from mandatory labeling [40].
Other fish allergens
Fish tropomyosin has been identified in Mozambique tilapia as a 32 kDa allergen (Ore m 4) [72]. Recently, IgE reactivity to fish tropomyosin has been reported in another study [73]. Various other IgE-binding fish proteins have been described. Their clinical relevance is not yet fully understood.
Ingestion of fish roe may also lead to allergic sensitization and clinical allergy. The allergen Onc k 5 (chum salmon) is vitellogenin, a 118 kDa protein and precursor of yolk proteins [74]. Roe allergens from different fish species are cross-reactive but there is no cross-reactivity to hen’s egg or fish muscle allergens.
Occupational fish allergens
Parvalbumins are important allergens in the fish-processing work environment where sensitization occurs via the skin or the respiratory tract. Other high molecular weight compounds seem to play also a role in patients with allergic rhinitis and asthma. Beyond parvalbumin, further allergens have been described for clinical case, including phaseolin, creatine kinase and α-actinin-3 [75,76,77,78]. Their diagnostic relevance is still unclear.
Component-resolved diagnosis: why and when
Diagnostic extracts
The current IgE-based diagnosis of fish-allergic patients (serum, skin) is based on the use of fish extracts (Table 1). Diagnostic food allergen extracts are known to be variable in content [5••]. These variations depend mainly on the food source used for protein extraction as well as the extraction protocol. The parvalbumin content in diagnostic skin extracts was reported to vary from 20 to 70 μg/ml of extract (no parvalbumin detectable in single samples) while the protein content ranged from 320 to 2270 μg/ml extract [57]. Thus, the ratio of parvalbumin to total protein varied greatly from 1 to 13.4%. Recently, diagnostic extracts were evaluated by antibody-based testing, in combination with a proteomics approach and IgE-binding analysis [79]. Distinct variations (up to factor 10) were found when comparing samples from different providers and fish species, in respect to total protein, allergen content and IgE recognition patterns. Many samples did not contain the complete spectrum of relevant allergens, including parvalbumin, enolase, aldolase and collagen suggesting diagnostic imprecision in the skin test when using the diagnostic extract.
Diagnostic IgE testing
Singleplex testing is currently available for quantification of IgE binding to a number of extracts (n = 28) as well as 2 allergens, recombinant parvalbumins from carp and cod (Table 1). Given the fact that hundreds of fish species are available on the market, it becomes clear that not complete spectra can be targeted but the use of representative fish extracts from relevant taxonomic orders.
Component-resolved testing, comparing a single allergen vs fish extracts, has been reported in several studies, mostly in the context of allergen identification. However, studies beyond IgE-binding measurement but integration of IgE data with food challenge data are scarce.
A single DBPCFC trial (total, n = 35) correlated so far the clinical reactivity to different fish (cod, salmon, mackerel) with IgE reactivity to extracts and single fish allergens, parvalbumin, enolase and aldolase, from the respective species [23••]. High diagnostic sensitivity was found for both IgE tests with fish extracts and parvalbumins. Extract-specific IgE (cod, salmon) discriminated best between individuals reacting to all fish vs those reacting to single/specific fish only, concluding that patients with particular IgE titers (cod, > 8.2 kUA/L; salmon, > 5 kUA/L) shall be advised to avoid any fish. Parvalbumins were confirmed as markers for clinical cross-reactivity [23••, 80]. However, they showed poor ability to identify patients with partial tolerance. Specific IgE to enolase and aldolase, together with anti-parvalbumin IgE, were concluded to be markers for more severe clinical reactions. This translational study demonstrated that both reagents are needed, fish extracts as well as the single molecules.
Other studies described that fish-allergic patients might have specific IgE, recognizing only the parvalbumin from specific fish, correlating with their clinical mono-sensitivity. Patients with salmonid fish allergy had IgE to salmon and trout parvalbumin fish only [30, 31]. Similarly, parvalbumin-specific IgE corresponding to species-specific clinical profiles were confirmed by others [33].
In a systematic approach, a panel of parvalbumins covering homologues from representative taxonomic groups might be advisable, in addition to the respective fish extracts. Such a parvalbumin selection is proposed in Table 1, amended by cod enolase and aldolase. Both cod and carp parvalbumin might not be required as they are highly cross-reactive [81]. Other fish allergens might be covered by the extracts but ideally, shall be included as further diagnostic molecules (e.g. collagen). Based on the variety of proposed diagnostic molecules, fish allergy is a showcase for future multiplex IgE testing in the format of in-vitro diagnostic platforms (e.g. ALEX, Macro Array Diagnostics; ImmunoCAP ISAC, Thermo Scientific). Finally, it is important to point out that the current state of knowledge on diagnostically relevant fish allergens is based on limited patients’ cohorts that are characterized by specific patterns of fish consumption and local diets.
Diagnostic challenges and research needs
Avoid or not?
Fish-allergic patients feature various levels of cross-reactivity (Fig. 1). Most react to many fish, sometimes with severe reactions, and shall be advised to avoid any type of fish [24]. A parvalbumin-positive IgE test, together with confirmation from the clinical history and possibly, skin tests with fresh fish, may address the diagnosis of those individuals.
Other patients have partial tolerances to specific fish and unnecessary avoidance of all fish should be prevented [23••]. However, a precise in-vitro diagnosis remains challenging in those cases. Parvalbumin-specific IgE tests are often false positive. Mostly allergens other than parvalbumins might play a role. Once the tolerated species is/are identified and the patient starts to introduce it/those in the diet, the question is whether the established tolerance is sustainable or whether the food allergy might spread to the tolerated species as well. Reference literature data on longitudinal patient follow-up upon reintroduction, including IgE-pattern development, are missing here.
Which fish?
Patients with parvalbumin-specific IgE only often tolerate low-parvalbumin fish such as tuna or swordfish [57, 58]. Systematic searches for further low-parvalbumin fish are lacking—they might reveal new dietary alternatives for patients with moderate to high threshold dose reactivity. Species distantly related to bony fish, such as cartilaginous fish (e.g. ray) expressing α-parvalbumins, might be consumed safely but this needs further clinical confirmation [29•]. For patients with suspected outgrown fish allergy, fish reintroduction schemes (from low to high allergenic fish) would be useful, together with corresponding specific IgE markers, to minimize food allergy recurrence and thus, to support the clinical management and patient follow-up. As an important practical note, when the consumption of specific fish is targeted, attention needs to be payed to the food product as species mislabeling does occur on the market [82].
Challenging basophils with fish?
The basophil activation test (BAT) emerged as a useful test in food allergy diagnosis, with similar sensitivity but superior specificity as compared with IgE tests (serum, skin) [83•]. Recently, BAT has been reported as being of importance to identify oral tolerance to ray in fish-allergic patients [29•]. Further studies on the clinical validation of BAT will be required in order to establish the diagnostic performance of this cellular assay in fish-allergic patients. Recommended study conditions include food challenge-proven allergy and the comparative testing of several allergens.
Conclusion
Fish-allergic patients are diverse in many ways, the severity of the clinical reaction including variable threshold doses, symptoms-eliciting species and the level of clinical cross-reactivity. Many fish allergens have been identified in the past decades contributing to a better understanding of the clinical reactivity profiles. Parvalbumin-specific IgE are markers for cross-reactions. Co-sensitization to parvalbumin and enolase/aldolase refers to severity of the clinical reaction. Fish extracts still represent an indispensable basis for in-vitro diagnosis. As such, molecular fish allergy diagnosis represents a showcase for in-vitro multiplex approaches with the overarching goal to avoid unnecessary food restrictions and thus, improve patient care.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ho MH, Wong WH, Chang C. Clinical spectrum of food allergies: a comprehensive review. Clin Rev Allergy Immunol. 2014;46(3):225–40.
Lopata AL, Jeebhay MF. Airborne seafood allergens as a cause of occupational allergy and asthma. Curr Allergy Asthma Rep. 2013;13(3):288–97.
Dickel H, Bruckner T, Altmeyer P, Künzlberger B. Seafood allergy in cooks: a case series and review of the literature. J Dtsch Dermatol Ges. 2014;12(10):891–902.
Rehbein H, Oehlenschläger J. Fishery products: quality, safety and authenticity. West Sussex: Wiley-Blackwell; 2009.
•• Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI molecular allergology user’s guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1–250 Benchmark on basics and allergens around component-resolved diagnosis af allergic patients.
• Stephen JN, Sharp MF, Ruethers T, Taki A, Campbell DE, Lopata AL. Allergenicity of bony and cartilaginous fish - molecular and immunological properties. Clin Exp Allergy. 2017;47(3):300–12 Comprehensive overview on parvalbumins from α- and β-lineage, including non-/allergens.
Agyemang A, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome: a comprehensive review. Clin Rev Allergy Immunol. 2019:1–11. https://doi.org/10.1007/s12016-018-8722-z.
Douros K, Tsabouri S, Feketea G, Grammeniatis V, Koliofoti EG, Papadopoulos M, et al. Retrospective study identified fish and milk as the main culprits in cases of food protein-induced enterocolitis syndrome. Acta Paediatr. 2019. https://doi.org/10.1111/apa.14779.
Infante S, Marco-Martín G, Sánchez-Domínguez M, Rodríguez-Fernández A, Fuentes-Aparicio V, Alvarez-Perea A, et al. Food protein-induced enterocolitis syndrome by fish: not necessarily a restricted diet. Allergy. 2018;73(3):728–32.
Lehane L, Olley J. Histamine fish poisoning revisited. Int J Food Microbiol. 2000;58(1-2):1–37.
Ruethers T, Taki AC, Johnston EB, Nugraha R, Le TTK, Kalic T, et al. Seafood allergy: a comprehensive review of fish and shellfish allergens. Mol Immunol. 2018;100:28–57.
Mattiucci S, Cipriani P, Levsen A, Paoletti M, Nascetti G. Molecular epidemiology of Anisakis and anisakiasis: an ecological and evolutionary road map. Adv Parasitol. 2018;99:93–263.
Mazzucco W, Raia DD, Marotta C, Costa A, Ferrantelli V, Vitale F, et al. Anisakis sensitization in different population groups and public health impact: a systematic review. PLoS One. 2018;13(9):e0203671.
Caraballo L, Coronado S. Parasite allergens. Mol Immunol. 2018;100:113–9.
Sastre J, Lluch-Bernal M, Quirce S, Arrieta I, Lahoz C, Del Amo A, et al. A double-blind, placebo-controlled oral challenge study with lyophilized larvae and antigen of the fish parasite, Anisakis simplex. Allergy. 2000;55(6):560–4.
Moonesinghe H, Mackenzie H, Venter C, Kilburn S, Turner P, Weir K, et al. Prevalence of fish and shellfish allergy: a systematic review. Ann Allergy Asthma Immunol. 2016;117(3):264–72.
Dunlop JH, Keet CA. Epidemiology of food allergy. Immunol Allergy Clin N Am. 2018;38(1):13–25.
Lyons SA, Burney PGJ, Ballmer-Weber BK, Fernandez-Rivas M, Barreales L, Clausen M, et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. 2019;7(6):1920–8.
Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098.
Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374(18):1733–43.
Klingberg S, Brekke HK, Ludvigsson J. Introduction of fish and other foods during infancy and risk of asthma in the All Babies In Southeast Sweden cohort study. Eur J Pediatr. 2019;178(3):395–402.
Vasileiadou S, Wennergren G, Strömberg Celind F, Åberg N, Pettersson R, et al. Eating fish and farm life reduce allergic rhinitis at the age of twelve. Pediatr Allergy Immunol. 2018;29(3):283–9.
•• Sørensen M, Kuehn A, Mills ENC, Costello CA, Ollert M, Småbrekke L, et al. Cross-reactivity in fish allergy: a double-blind, placebo-controlled food-challenge trial. J Allergy Clin Immunol. 2017;140(4):1170–2 First double-blind placebo-controlled study approaching the correlation between clinical reactivity and molecular IgE-profiles in fish-allergic patients.
Pouessel G, Turner PJ, Worm M, Cardona V, Deschildre A, Beaudouin E, et al. Food-induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. 2018;48(12):1584–93.
Helbling A, Haydel R Jr, McCants ML, Musmand JJ, El-Dahr J, Lehrer SB. Fish allergy: is cross-reactivity among fish species relevant? Double-blind placebo-controlled food challenge studies of fish allergic adults. Ann Allergy Asthma Immunol. 1999;83:517–23.
Sten E, Hansen TK, Stahl Skov P, Andersen SB, Torp A, Bindslev-Jensen U, et al. Cross-reactivity to eel, eelpout and ocean pout in codfish-allergic patients. Allergy. 2004;59(11):1173–80.
Schulkes KJ, Klemans RJ, Knigge L, de Bruin-Weller M, Bruijnzeel-Koomen CA, Marknell deWitt A, et al. Specific IgE to fish extracts does not predict allergy to specific species within an adult fish allergic population. Clin Transl Allergy. 2014;4:27.
Kobayashi Y, Huge J, Imamura S, Hamada-Sato N. Study of the cross-reactivity of fish allergens based on a questionnaire and blood testing. Allergol Int. 2016;65(3):272–9.
• Kalic T, Morel-Codreanu F, Radauer C, Ruethers T, Taki AC, Swoboda I, et al. Patients allergic to fish tolerate ray based on the low allergenicity of its parvalbumin. J Allergy Clin Immunol Pract. 2019;7(2):500–508.e11 First study on basophil activation test in fish allergy diagnosis.
Kuehn A, Hutt-Kempf E, Hilger C, Hentges F. Clinical monosensitivity to salmonid fish linked to specific IgE-epitopes on salmon and trout beta-parvalbumins. Allergy. 2011;66(2):299–301.
Vázquez-Cortés S, Nuñez-Acevedo B, Jimeno-Nogales L, Ledesma A, Fernández-Rivas M. Selective allergy to the Salmonidae fish family: a selective parvalbumin epitope? Ann Allergy Asthma Immunol. 2012;108(1):62–3.
Kuehn A, Fischer J, Hilger C, Sparla C, Biedermann T, Hentges F. Correlation of clinical monosensitivity to cod with specific IgE to enolase and aldolase. Ann Allergy Asthma Immunol. 2014;113(6):670–1.
Raith M, Klug C, Sesztak-Greinecker G, Balic N, Focke M, Linhart B, et al. Unusual sensitization to parvalbumins from certain fish species. Ann Allergy Asthma Immunol. 2014;113(5):571–2.
Viñas M, Pineda F, Izquierdo-Domínguez A, Castillo M, Castillo MJ, Hernández N, et al. Allergy to Limanda aspera (yellowfin sole): report of a case of food allergy in a child. J Investig Allergol Clin Immunol. 2018;28(2):137–8.
Valverde-Monge M, Pastor-Vargas C, Rodríguez Del Rio P, Escudero C, Sánchez-García S, Mendez Brea P, et al. Anaphylaxis by exclusive allergy to swordfish and identification of a new fish allergen. Pediatr Allergy Immunol. 2018;29(5):563–5.
Kondo Y, Komatsubara R, Nakajima Y, Yasuda T, Kakami M, Tsuge I, et al. Parvalbumin is not responsible for cross-reactivity between tuna and marlin: a case report. J Allergy Clin Immunol. 2006;118(6):1382–3.
Ebo DG, Kuehn A, Bridts CH, Hilger C, Hentges F, Stevens WJ. Monosensitivity to pangasius and tilapia caused by allergens other than parvalbumin. J Investig Allergol Clin Immunol. 2010;20(1):84–8.
Kuehn A, Hilger C, Lehners-Weber C, Codreanu-Morel F, Morisset M, Metz-Favre C, et al. Identification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergens. Clin Exp Allergy. 2013;43(7):811–22.
Ballmer-Weber BK, Fernandez-Rivas M, Beyer K, Defernez M, Sperrin M, Mackie AR, et al. How much is too much? Threshold dose distributions for 5 food allergens. J Allergy Clin Immunol. 2015;135(4):964–71.
Kourani E, Corazza F, Michel O, Doyen V. What we know about fish allergy by the end of the decade? J Investig Allergol Clin Immunol. 2019. https://doi.org/10.18176/jiaci.0381.
Taylor SL, Baumert JL. Worldwide food allergy labeling and detection of allergens in processed foods. Chem Immunol Allergy. 2015;101:227–34.
Jeebhay MF, Moscato G, Bang BE, Folletti I, Lipińska-Ojrzanowska A, Lopata AL, et al. Food processing and occupational respiratory allergy-A EAACI Position Paper. Allergy. 2019. https://doi.org/10.1111/all.13807.
Fukutomi Y. Occupational food allergy. Curr Opin Allergy Clin Immunol. 2019;19(3):243–8.
Dahlman-Höglund A, Renström A, Acevedo F, Andersson E. Exposure to parvalbumin allergen and aerosols among herring processing workers. Ann Occup Hyg. 2013;57(8):1020–9.
Kaae J, Menné T, Thyssen JP. Severe occupational protein contact dermatitis caused by fish in 2 patients with filaggrin mutations. Contact Dermatitis. 2013;68(5):319–20.
Kuehn A, Swoboda I, Arumugam K, Hilger C, Hentges F. Fish allergens at a glance: variable allergenicity of parvalbumins, the major fish allergens. Front Immunol. 2014;5:179.
Swoboda I, Balic N, Klug C, Focke M, Weber M, Spitzauer S, et al. A general strategy for the generation of hypoallergenic molecules for the immunotherapy of fish allergy. J Allergy Clin Immunol. 2013;132(4):979–81.
Zuidmeer-Jongejan L, Huber H, Swoboda I, Rigby N, Versteeg SA, Jensen BM, et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol. 2015;166(1):41–51.
Freidl R, Gstöttner A, Baranyi U, Swoboda I, Stolz F, Focke-Tejkl M, et al. Resistance of parvalbumin to gastrointestinal digestion is required for profound and long-lasting prophylactic oral tolerance. Allergy. 2019. https://doi.org/10.1111/all.13994.
Bugajska-Schretter A, Elfman L, Fuchs T, Kapiotis S, Rumpold H, Valenta R, et al. Parvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletion. J Allergy Clin Immunol. 1998;101:67–74.
Ruethers T, Raith M, Sharp MF, Koeberl M, Stephen JN, Nugraha R, et al. Characterization of Ras k 1 a novel major allergen in Indian mackerel and identification of parvalbumin as the major fish allergen in 33 Asia-Pacific fish species. Clin Exp Allergy. 2018;48(4):452–63.
Saptarshi SR, Sharp MF, Kamath SD, Lopata AL. Antibody reactivity to the major fish allergen parvalbumin is determined by isoforms and impact of thermal processing. Food Chem. 2014;148:321–8.
Van Do T, Elsayed S, Florvaag E, Hordvik I, Endresen C. Allergy to fish parvalbumins: studies on the cross-reactivity of allergens from 9 commonly consumed fish. J Allergy Clin Immunol. 2005;116:1314–20.
Hilger C, Thill L, Grigioni F, Lehners C, Falagiani P, Ferrara A, et al. IgE antibodies of fish allergic patients cross-react with frog parvalbumin. Allergy. 2004;59(6):653–60.
Kuehn A, Codreanu-Morel F, Lehners-Weber C, Doyen V, Gomez-André SA, Bienvenu F, et al. Cross-reactivity to fish and chicken meat - a new clinical syndrome. Allergy. 2016;71(12):1772–81.
Ballardini N, Nopp A, Hamsten C, Vetander M, Melén E, Nilsson C, et al. Anaphylactic reactions to novel foods: case report of a child with severe crocodile meat allergy. Pediatrics. 2017;139(4):e20161404.
Kuehn A, Scheuermann T, Hilger C, Hentges F. Important variations in parvalbumin content in common fish species: a factor possibly contributing to variable allergenicity. Int Arch Allergy Immunol. 2010;153(4):359–66.
Griesmeier U, Vázquez-Cortés S, Bublin M, Radauer C, Ma Y, Briza P, et al. Expression levels of parvalbumins determine allergenicity of fish species. Allergy. 2010;65(2):191–8.
Schrama D, Cerqueira M, Raposo CS, Rosa Da Costa AM, Wulff T, Gonçalves A, et al. Dietary creatine supplementation in gilthead seabream (Sparus aurata): comparative proteomics analysis on fish allergens, muscle quality, and liver. Front Physiol. 2018;9:1844.
Sletten G, Van Do T, Lindvik H, Egaas E, Florvaag E. Effects of industrial processing on the immunogenicity of commonly ingested fish species. Int Arch Allergy Immunol. 2010;151(3):223–36.
Yang H, Min J, Han XY, Li XY, Hu JW, Liu H, et al. Reduction of the histamine content and immunoreactivity of parvalbumin in Decapterus maruadsi by a Maillard reaction combined with pressure treatment. Food Funct. 2018;9(9):4897–905.
Sánchez R, Martínez J, Castro A, Pedrosa M, Quirce S, Rodríguez-Pérez R, et al. The amyloid fold of Gad m 1 epitopes governs IgE binding. Sci Rep. 2016;6:32801.
Liu R, Krishnan HB, Xue W, Liu C. Characterization of allergens isolated from the freshwater fish blunt snout bream (Megalobrama amblycephala). J Agric Food Chem. 2011;59:458–63.
Tomm JM, van Do T, Jende C, Simon JC, Treudler R, von Bergen M, et al. Identification of new potential allergens from nile perch (Lates niloticus) and Cod (Gadus morhua). J Investig Allergol Clin Immunol. 2013;23:159–67.
Rosmilah M, Shahnaz M, Meinir J, Masita A, Noormalin A, Jamaluddin M. Identification of parvalbumin and two new thermolabile major allergens of Thunnus tonggol using a proteomics approach. Int Arch Allergy Immunol. 2013;162(4):299–309.
Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009;23:563–76.
Hamada Y, Nagashima Y, Shiomi K. Identification of collagen as a new fish allergen. Biosci Biotechnol Biochem. 2001;65:285–91.
Kuehn A, Hilger C, Hentges F. Anaphylaxis provoked by ingestion of marshmallows containing fish gelatin. J Allergy Clin Immunol. 2009;123:708–9.
Kobayashi Y, Kuriyama T, Nakagawara R, Aihara M, Hamada-Sato N. Allergy to fish collagen: thermostability of collagen and IgE reactivity of patients’ sera with extracts of 11 species of bony and cartilaginous fish. Allergol Int. 2016;65:450–8.
Kobayashi Y, Akiyama H, Huge J, Kubota H, Chikazawa S, Satoh T, et al. Fish collagen is an important panallergen in the Japanese population. Allergy. 2016;71(5):720–3.
Allergy to fish collagen: thermostability of collagen and IgE reactivity of patients’ sera with extracts of 11 species of bony and cartilaginous fish. Allergol Int. 2016;65(4):450–8.
Liu R, Holck AL, Yang E, Liu C, Xue W. Tropomyosin from tilapia (Oreochromis mossambicus) as an allergen. Clin Exp Allergy. 2013;43:365–77.
González-Fernández J, Alguacil-Guillén M, Cuéllar C, Daschner A. Possible allergenic role of tropomyosin in patients with adverse reactions after fish intake. Immunol Investig. 2018;47:416–29.
Shimizu Y, Kishimura H, Kanno G, Nakamura A, Adachi R, Akiyama H, et al. Molecular and immunological characterization of β'-component (Onc k 5), a major IgE-binding protein in chum salmon roe. Int Immunol. 2014;26(3):139–47.
Sano A, Yagami A, Suzuki K, Iwata Y, Kobayashi T, Arima M, et al. Two cases of occupational contact urticaria caused by percutaneous sensitization to parvalbumin. Case Rep Dermatol. 2015;7(2):227–32.
Yagami A, Suzuki K, Nakamura M, Sano A, Kobayashi T, Iwata Y. at al. Occupational food allergy due to parvalbumin and phaseolin induced by epicutaneous sensitization. Allergol Int. 2015;64(3):287–8.
Larco-Rojas X, González-Gutiérrez ML, Vázquez-Cortés S, Bartolomé B, Pastor-Vargas C, Fernández-Rivas M. Occupational asthma and urticaria in a fishmonger due to creatine kinase, a cross-reactive fish allergen. J Investig Allergol Clin Immunol. 2017;27(6):386–8.
Shimojo N, Yagami A, Nakamura M, Nagai A, Matsunaga K. Occupational fish allergy caused by percutaneous sensitization with α-actinin-3. Contact Dermatitis. 2017;76(5):322–3.
Ruethers T, Taki AC, Nugraha R, Cao TT, Koeberl M, Kamath SD, et al. Variability of allergens in commercial fish extracts for skin prick testing. Allergy. 2019. https://doi.org/10.1111/all.13748.
Hilger C, van Hage M, Kuehn A. Diagnosis of allergy to mammals and fish: cross-reactive vs. specific markers. Curr Allergy Asthma Rep. 2017;17(9):64.
Vázquez-Cortés S, Eguiluz Gracia I, Radauer C, Breiteneder H, Fernández-Rivas M. Diagnostic performance of single and multiplex IgE testing to recombinant parvalbumins in fish allergy. Ann Allergy Asthma Immunol. 2012;109(5):362–3.
Mazzeo MF, Siciliano RA. Proteomics for the authentication of fish species. J Proteome. 2016;147:119–24.
• Santos AF, Shreffler WG. Road map for the clinical application of the basophil activation test in food allergy. Clin Exp Allergy. 2017;47(9):1115–24 Comprehensive overview on basophil activations tests, including perspectives and limitations, in food allergy diagnosis.
Haroun-Díaz E, Blanca-López N. Vázquez de la Torre M, Ruano FJ, Somoza Álvarez ML, Labrador Horrillo M, et al. Severe anaphylaxis due to crocodile-meat allergy exhibiting wide cross-reactivity with fish allergens. J Allergy Clin Immunol Pract. 2018;6(2):669–70.
Funding
This work was supported by the Ministry of Research, Luxembourg (JK, AK), the PRIDE program grant (PRIDE/11012546/NEXTIMMUNE) by the Fonds National de la Recherche (FNR), Luxembourg (JK, AK) and the Personalized Medicine Consortium Luxembourg (JK, AK). This work received national funds through FCT - Foundation for Science and Technology through project UID/Multi/04326/2019. DS acknowledges FCT PhD grant, Refª Refª SFRH/BD/136319/2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Julia Klueber declares that she has no conflicts of interest. Denise Schrama declares that she has no conflicts of interest. Pedro Rodrigues declares that he has no conflicts of interest. Heinrich Dickel declares that he has no conflicts of interest. Annette Kuehn declares that she has no conflicts of interest.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Food Allergy
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Klueber, J., Schrama, D., Rodrigues, P. et al. Fish Allergy Management: From Component-Resolved Diagnosis to Unmet Diagnostic Needs. Curr Treat Options Allergy 6, 322–337 (2019). https://doi.org/10.1007/s40521-019-00235-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-019-00235-w