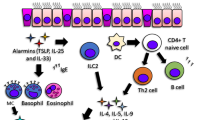
Opinion statement
Several recent studies spanning from induced sputum to gene signature identified non-eosinophilic asthma (NEA) as a distinct asthma phenotype with several relevant clinical features such as increased asthma severity, increased remodelling and lower response to bronchodilator and anti-inflammatory treatment. Two major mechanisms leading to neutrophilic inflammation are postulated: the dysregulated innate immune response, including neutrophil intrinsic abnormalities, and the activation of the IL-17-dependent pathway. Several factors such as age, metabolic or epigenetic factors or the activation of the epithelial-mesenchimal trophic unit have been identified as modulators. The endotyping of NEA is far behind the eosinophilic asthma, and until now, no endotype-driven interventions have been proved to be effective.
Similar content being viewed by others
Non-eosinophilic asthma as a distinct asthma phenotype
Using induced sputum, four distinct inflammatory subtypes of asthma were described: eosinophilic, neutrophilic, paucigranulocytic and mixed granulocytic [1•]. Studies in large cohorts, such as the SARP (Severe Asthma Research Program) cohort, identified neutrophilic inflammation through induced sputum as an important hallmark of a distinct cluster of patients with moderate to severe asthma [2]. More recent studies evaluating the molecular signature of the Th2 genes [3] or using unsupervised hierarchical clustering of gene expression profiles in asthma [4] reinforce the reality of non-eosinophilic asthma (NEA).
Based on data from Th2 high/low molecular signature studies, the incidence of adult NEA reaches 30–50 % [3]. In the Asthma Clinical Research Network, 47 % subjects with mild to moderate asthma were persistently non-eosinophilic [5]. In addition, almost half of the subjects with mild asthma exacerbations had no sputum eosinophilia [6]. In children, data are scarce and controversial, and the stability of the non-eosinophilic pattern has been challenged [7]. Older studies showed that the pattern of inflammatory phenotypes differs between adults and children, with eosinophilic inflammation being more prevalent in both acute and stable childhood asthma, and neutrophilic inflammation being the dominant pattern of acute asthma in adults [8]. Latter studies report incidence of NEA in 30 % of steroid-naive asthmatic children and in 37.5 % of children with severe-persistent asthma is [9, 10]. In obese children with asthma the incidence of NEA was significantly higher only amongst females [11].
There are several important clinical features that distinguish eosinophilic from NEA. An observational cross-sectional study on adults with asthma compared lung function, asthma control, exacerbation frequency and the type of co-morbidities between eosinophilic, neutrophilic and paucigranulocytic asthma. Pre- and post-bronchodilator FEV1 percentage predicted was highest in paucigranulocytic asthma and lowest in neutrophilic and eosinophilic asthma, but forced inspiratory flow (FIF50) tended to be lowest in neutrophilic asthma with a significant inverse correlation between sputum neutrophils and FIF50 percentage predicted. Patients with neutrophilic asthma had a significant lower bronchodilator response compared with eosinophilic asthma, while those with paucigranulocytic asthma tended to have less responsive airways. Patients with eosinophilic asthma had the poorest asthma control. The frequencies of severe exacerbations were similar across inflammatory subtypes; however, patients with paucigranulocytic and neutrophilic asthma had more than double the amount of primary care visits for uncontrolled asthma in the past year. There was no difference in smoking histories between patients of different inflammatory subtypes, and no difference in FEV1/FVC ratio; thus, smoking history or the presence of mild or co-existing COPD is not a distinguishing feature of neutrophilic asthma [12•]. Both in children and adults, sputum neutrophils correlated with asthma severity and with the extent of remodelling [10, 13]. In addition, most of the observations describe NEA as less responsive to currently available anti-inflammatory therapy [3, 5, 10, 14].
The concept of asthma complex endotypes
Asthma is a heterogeneous syndrome that covers a broad spectrum of complex genetic, inflammatory, metabolic and remodelling networks that lead to several pathogenetic pathways. Classifying asthma based on underlying pathophysiologic mechanisms, known as endotyping, offers a stratified approach for a better assessment of disease epidemiology, genetic background and environmental risk factors, for the development of new targeted therapies for asthma and for a better selection of responders to targeted treatment [15, 16, 17•]. Single molecular mechanism-linked endotypes can be defined for asthma; however, most asthma endotypes involve concomitantly several pathways. Examples of such complex endotypes are Th2 inflammation or aspirin-intolerant asthma [18••].
The “Holy Grail” of asthma endotyping is to pinpoint the essential nodes of the network that are unique for one endotype and relate to relevant clinical end-points such as disease severity or response to treatment. For Th2-asthma endotype, three such nodes can be described, each depicting a distinct subendotype: the IL-5 pathway, the IL-4/IL-13 pathway and the IgE pathway [18••].
Animal models for non-eosinophilic asthma
Aligning mouse models of asthma to human endotypes of disease is essential for describing better the underlying networks of various asthma endotypes. In the quest to describe the mechanisms of NEA, several mouse models have been developed that allow the study of airway hyperreactivity (AHR), extensive remodelling, Th17-driven inflammation, association with obesity or corticosteroid unresponsiveness [19–22].
Another animal model for neutrophilic asthma is the recurrent airway obstruction (RAO) or heaves occurring in horses. RAO is a common, performance-limiting, equine respiratory disease triggered by dust components such as endotoxin with clinical signs and pathophysiological similarities to human asthma, characterised by bronchospasm, neutrophilic infiltration and increased mucus in the airways. Neutrophils accumulate in the airways and their chemotaxis is regulated via MAPK and PI3K pathways [23]. In addition, expression of IL1β, IL8, TLR4, TNFα, TGFβ1 and NFkβ transcripts is significantly up-regulated and is correlated with clinical features [24].
Neutrophilic inflammation in asthma: triggers, mechanisms and potential subendotypes
The mechanisms driving a neutrophilic predominant inflammation in asthma are not fully elucidated.
Potential triggers of neutrophilic inflammation include infection, gastroesophageal reflux disease (GERD) with aspiration and rhinosinusitis. In the same observational study mentioned above [12•], the frequency of chest infections and the incidence of chronic rhinosinusitis and of GERD were significantly higher in patients with neutrophilic asthma. In another trial including subjects with stable asthma, a significant load of potentially pathogenic bacteria was cultured from the sputum and was associated with higher total cell counts, proportion and number of neutrophils, sputum IL-8 and 8-isoprostane concentrations [25]. Bacterial components in indoor dust, such as nanometre-sized extracellular vesicles, can evoke neutrophilic inflammation as well. In a mouse model of asthma, repeated intranasal application of indoor dust induced neutrophilic pulmonary inflammation accompanied by lung infiltration with both Th1 and Th17 cells [26].
Air pollution may also be a prominent trigger of neutrophilic bronchitis. In a Canadian retrospective study, patients with the highest sputum neutrophils were living within 1000 m of highways [27].
Usually, significantly higher doses of inhaled corticosteroids (ICS) are needed to control neutrophilic asthma. ICS are reported to increase neutrophil survival and therefore may have a direct influence on sputum neutrophil proportions.
Two major pathways seem to be involved in the pathogenesis of neutrophilic asthma: an altered innate immune response and the activation of Th17 specific immune response. Each of these pathways is further modulated by age and by interaction with external triggers such as infections or pollution and by metabolic or epigenetic factors. The input from the resident cells, especially in the remodelled status, is further fine-tuning the neutrophilic inflammatory response. In contrast to Th2 high asthma, the translation into specific disease subendotypes has not been done yet since there are very few targeted interventions for neutrophilic asthma, none of them with a significant effect in large clinical trials.
A. The dysregulated innate immune response in neutrophilic asthma
Growing evidence supports a role for a dysregulated innate immune response in neutrophilic asthma, with altered gene expression of Toll-like receptors (TLR) and increased expression of genes from the IL-1β and TNF-α/nuclear factor-κB pathways [28], phenotype switch and enhanced neutrophil chemotaxis and survival in the airways, impairment in anti-inflammatory mechanisms and in pathogen recognition and destruction processes and increased protease activity.
A1 The IL-1β and TNF-α/nuclear factor-κB pathway
The recently described six-gene signature that distinguishes between eosinophilic and non-eosinophilic asthma includes three genes involved in innate immune response that all are associated with NEA: IL-1β, alkaline phosphatase, tissue non-specific isozyme (ALPL) and chemokine (C-X-C motif) receptor 2 (CXCR2) [29•].
In an experimental model of Th1/neutrophil-predominant asthma, TNFα reduced the responsiveness to steroids and neutralising TNFα using etanercept restored glucocorticoid sensitivity [30]. Recently, the tumour necrosis factor superfamily member, LIGHT, has been recognised as a key mediator in severe asthmatic airway inflammation. Human bronchial epithelial cells (BEC) express one of the receptors for LIGHT, the lymphotoxin β receptor (LTβR). Stimulation of BEC with LIGHT induced IL-6, oncostatin M, MCP-1, growth-regulated protein α and IL-8.
Specific siRNA for LTβR attenuated IL-6 and IL-8 production. LIGHT activated intracellular signalling, such as mitogen-activated protein kinase and nuclear factor-κB (NF-κB) signalling [31].
A2 Neutrophil abnormalities
Neutrophils are important in innate immunity, and their numbers are increased in the airways in NEA. The activity of neutrophils differs in relation with the predominant inflammatory asthma phenotype. In NEA, blood neutrophils release significantly higher levels of interleukin-8 at rest. Cytokine gene expression and sputum neutrophil protein production did not differ between asthma subtypes. However, microarrays demonstrated expression profiles for NEA that were significantly distinct from eosinophilic asthma. A total of 317 genes were significantly altered in resting neutrophils from NEA, including genes related to cell motility and regulation of apoptosis supporting further the enhancement of blood neutrophil chemotaxis and survival as a major mechanism of NEA [32]. Persistent elevation of airway neutrophils due to impaired efferocytosis was also described. Galectin-3 (gal-3), a member of the ß-galactoside-binding animal lectins, involved in the recruitment, activation and removal of neutrophils, is significantly reduced in neutrophilic asthma compared with eosinophilic and paucigranulocytic asthma. In addition, patients with neutrophilic asthma have impairment in anti-inflammatory ratio of gal-3/gal-3-binding protein and IL-1RA/IL-1β [33].
Furthermore, neutrophils can switch to an N2 phenotype, contributing to the pool of IL-13, which in addition to IL-4, promotes activation of macrophages limiting further injury and promoting repair [34••].
A3 Other mechanisms
Altered pathogen recognition and destruction processes are present with macrophage efferocytosis impaired in NEA to a similar degree as in COPD [35]. Proteases are important mediators of inflammation, cytokine activation and tissue remodelling. Subjects with eosinophilic asthma had significantly high levels of active MMP-9 compared with those with neutrophilic asthma. Although, there are high levels of total MMP-9 in neutrophilic asthma, most of it is inactivated and bound to the tissue inhibitor of MMP. On the other hand, in neutrophilic asthma, there is increased activity of neutrophil elastase (NE), in direct correlation with the levels of interleukin-8 and neutrophils [36].
B. The IL-17 pathway
IL-17, through the induction of cytokines and chemokines, is the major driving force for the recruitment and activation of neutrophils. IL-17 is mainly secreted by Th17 cells, a distinct CD4T helper cell subset, characterised by the specific expression of the transcription factor retinoic acid-related orphan receptor-γt (RORγt) [37]. By secreting IL-17, Th17 cells orchestrate the recruitment of neutrophil granulocytes in the lungs and their activation directly through CXCL8 production or indirectly by inducing the production of IL-6, G-CSF and GM-CSF, IL-8, CXCL1 and CXCL5 by airway epithelial cells. Although Th17 cells represent a core source of IL-17, a newly identified population of innate lymphoid cells (ILCs) is also able to produce IL-17 [37, 38]. IL-17-producing ILC3s express CC chemokine receptor 6, produce IL-17 and sometimes IL-22, and are central to the development of asthma in obese mice. Increased numbers of this subset of cells have been found in the bronchoalveolar lavage (BAL) fluid of obese individuals with severe asthma [39].
B1. IL-17 and neutrophilic asthma
A likely role of IL-17 in neutrophilic asthma is suggested by the significant association between IL-17 and other diseases with underlying neutrophilic inflammation, such as psoriasis, and by the efficiency of IL-17-targeted therapies in these diseases [40]. The link between neutrophilic inflammation and Th17 immunity is well established in mouse models of asthma, where it has been linked to the development of AHR and remodelling [41, 42]. Human data are also accumulating; however, a cause and effect relationship between IL-17 production and neutrophilic asthma has not been demonstrated. A strong correlation between IL-17 and neutrophils was established in induced sputum and blood [43, 44]. IL-17 has been linked to remodelling, AHR asthma severity and inflammation [44–47]. In the Clinical Asthma Research Association cohort study including steroid-naive asthmatic children and healthy controls, patients with non-allergic asthma showed very distinctly IL-17-shifted proinflammatory immunity, promoting neutrophil inflammation and less functional Treg cells. In parallel, expressions of anti-inflammatory IL-37, proline-serine-threonine phosphatase-interacting protein 2, the neutrophil-associated genes CD93, triggering receptor expressed on myeloid cells 1, and regulator of G-protein signalling 13 were increased in patients [48]. The IL-17 pathway was also related to decreased corticosteroid sensitivity, also a characteristic of neutrophilic asthma [49].
B2. Co-factors for IL-17 induced neutrophilic inflammation
A mixed Th17/Th2 activation is frequently observed in asthma. The relationship between IL-17 and Th2-type immunity is highly complex. Th2 cells can differentiate into dual-positive Th2/Th17 cells [50] and these cells were identified in the BAL fluid of asthmatic patients [51]. The subgroup of asthmatic patients with dual-positive Th2/Th17 cells had glucocorticoid resistance in vitro and had the greatest airway obstruction and hyperreactivity [51•]. An interesting model suggests that IL-17 produced by γδ T cells and by Th17 cells in response to injured epithelium enhances the production of IL-4 and IL-13 from ILC2 and Th2 cells, which acts as a negative feedback loop shutting down further IL-17 production and neutrophilia [52]. Conversely, IL-4 and Il-13 may amplify Th17 responses by up-regulating CD209a expression on dendritic cells [53]. When the type-2 response fails to fully suppress the IL-17 response, or further promotes it, excessive tissue damage can occur. Understanding what regulates the IL-17–IL-4/13 feedback loops will provide invaluable insight into situations in which IL-17 exacerbates type-2 inflammation. Chitinases and chitinase-like proteins (CLPs) may be an important link between type-2 immunity and IL-17. Acting as danger-associated molecular patterns, CLPs release IL-1 with IL-17 production by innate γδ T cells, which in turn drives a type-2 response. Subsequently, type-2 cytokines induce even more CLP expression, which likely further contribute to repair. Additionally, CLPs can promote Th2 differentiation and suppress IFNγ, further promoting type-2 immunity [54].
IL-33 is another co-factor that cooperates with IL-17A to exacerbate AHR by enhancing neutrophilic inflammation via CXCR2 signalling. Furthermore, CXCR2 signalling derived Th2 responses are enhanced [55].
C. Modulators of the non-eosinophilic asthma endotypes
C1. Immune factors
The innate immune response has a critical role in the development of a specific immune response. For example, TLR4 expression by haematopoietic cells is critical for neutrophilic airway inflammation following LPS exposure and for Th17-driven neutrophilic responses to the house dust mite lysates and ovalbumin, while TLR4 expression by airway epithelial cells was found to be important for robust eosinophilic airway inflammation following sensitization and challenge with these same allergens [56].
An imbalance in Th17/Treg in peripheral blood was reported in NEA adult patients undergoing ICS treatment [57] in association with increased severity of the disease. The same observation was reported for paediatric asthma [48].
A subgroup NEA responds to inhaled steroids. An experimental model suggested that non-eosinophilic asthma associated predominantly with Th1/Tc1 cells is susceptible to glucocorticoid treatment and has no goblet cell metaplasia [58].
C2. Epigenetic factors
MicroRNAs (miRs) are small noncoding RNAs that regulate the function of immune cells by controlling mRNA stability and translation. MiR-9 expression is increased in sputum of patients with neutrophilic but not those with eosinophilic asthma. Exposure of pulmonary macrophages to IFN-γ/LPS synergistically induced miR-9 expression and reduced levels of its target transcript, protein phosphatase 2 regulatory subunit B (B56) δ isoform. Increased miR-9 attenuated PP2A activity and inhibited the nuclear translocation of the glucocorticoid receptor (GR). Inhibition of miR-9 increased both PP2A activity and GR nuclear translocation in macrophages and restored steroid sensitivity in multiple models of steroid-resistant AHR [59].
Airway smooth muscle (ASM) cells isolated from asthmatic individuals secrete more CXCL8 than cells from non-asthmatic individuals. CXCL8 is a CXC chemokine that drives steroid-resistant neutrophilic airway inflammation. CXCL8 increased secretion is due to increased histone H3 acetylation and increased binding of histone acetyltransferase to the CXCL8 promoter complex. The epigenetic defect can be modulated by Brd inhibitors that may provide a new therapeutic strategy for steroid-resistant inflammation [60].
C3. Ageing and non-eosinophilic asthma
A significant high incidence of asthma severity was reported for elderly patients [61]. Airway neutrophilia is related to age in adults, with a neutrophilic asthma phenotype present in older adults [62•].
Therapeutic implications
Correct identification and correction of co-morbidities such as infections, CRS and GERD might prove successful as a first step to control non-eosinophilic asthma. Reversal of steroid resistance is also a reasonable approach for severe cases. Targeting remodelling or switching-off the secretory phenotype of ASM is still experimental.
In the AZISAST study azithromycin did not reduce the rate of severe exacerbations and lower respiratory tract infections in all patients with severe asthma but had a significant effect in subjects with non-eosinophilic severe asthma defined by blood eosinophilia ≤200/μl [63]. A recent meta-analysis of 18 trials and 1306 asthmatics treated with macrolides showed differential effects according to race: macrolides increased FEV1 and PEF in Caucasians and Asians, decreased AHR in Caucasian, and improved cell counts of sputum amongst Asians. There was no effect on symptom scores or quality of life [64]. The longitudinal effect of macrolides on lung microbioma was evaluated in adult patients with moderate/severe asthma before and after 6 weeks of daily 250 mg azithromycin therapy. Azithromycin decreased bacterial richness in the airways and altered the airway microbiota leading to Anaerococcus becoming dominant within the bacterial community, while Pseudomonas, Haemophilus and Staphylococcus (three pathogenic genera associated with airway disease) were all reduced. In addition, azithromycin decreased mucus secretion and airway neutrophil accumulation as well as specific antibiotic and antipseudomonal activity [65•]. Macrolide antibiotics have been reported to be effective in the treatment of the neutrophilic asthma for reasons unrelated to their antibacterial action [66]. This has resulted in research activities aimed at gaining a better understanding of the immunomodulatory actions of macrolides and the synthesis of various novel anti-inflammatory macrolides without antimicrobial activity. Further work, including proteomic, genomic and microbiome studies, will advance our knowledge on the macrolide-responsive non-eosinophilic asthma endotype.
None of the targeted interventions for non-eosinophilic asthma proved successful in large clinical trials. One might argue that patient selections was not ideal and certainly did not follow the endotype-driven classification like it was done for Th2 asthma.
There is only one trial in asthma investigating the anti IL-17 pathway inhibition with brodalumab in subjects with inadequately controlled moderate to severe asthma taking regular inhaled corticosteroids. Inhibition of IL-17 receptor A did not produce a treatment effect in the over-all group. In the high-reversibility subgroup (post-bronchodilator FEV1 improvement ≥20 %) there was a significant ACQ improvement only in the low-dose group, but the effect was not observed in the higher-dose group, so the effect is of uncertain clinical significance [67•].
Strategies using small molecule antagonists against the interleukin-8 receptor, CXCR2, are able to reduce airway neutrophilia, and proved clinical efficacy in small proof-of-concept trials [68] but failed in large phase III clinical trials. A new series of monocyclic CXCR2 antagonists with improved solubility and good pharmacokinetic profiles is under investigation [69].
Conclusion
The progress in the endotyping of non-eosinophilic asthma is well behind the eosinophilic asthma. Several small steps have been taken but no clear subendotypes can be pinpointed. Until results from the large clinical trials with targeted endotype-driven interventions become available, the reliable identification and reduction of potential triggers such as infections, CRS, GERD or pollution exposure remains the recommended approach for non-eosinophilic asthma.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. Pivotal study describing the major inflammatory phenotypes of asthma using induced sputum.
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National heart, lung, and blood institute’s severe asthma research program. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–23.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
Baines KJ, Simpson JL, Wood LG, Scott RJ, Gibson PG. Transcriptional phenotypes of asthma defined by gene expression profiling of induced sputum samples. J Allergy Clin Immunol. 2011;127(1):153–60.
McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, et al. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–9.
Turner MO, Hussack P, Sears MR, Dolovich J, Hargreave FE. Exacerbations of asthma without sputum eosinophilia. Thorax. 1995;50:1057–61.
Fleming L, Tsartsali L, Wilson N, Regamey N, Bush A. Sputum inflammatory phenotypes are not stable in children with asthma. Thorax. 2012;67(8):675–81.
Wang F, He XY, Baines KJ, Gunawardhana LP, Simpson JL, Li F, et al. Different inflammatory phenotypes in adults and children with acute asthma. Eur Respir J. 2011;38(3):567–74.
Lee YJ, Kim KW, Choi BS, Sohn MH, Kim KE. Clinical characteristics of eosinophilic and noneosinophilic asthma in children. Acta Paediatr. 2013;102(1):53–7.
O’Brien CE, Tsirilakis K, Santiago MT, Goldman DL, Vicencio AG. Heterogeneity of lower airway inflammation in children with severe-persistent asthma. Pediatr Pulmonol. 2015. doi:10.1002/ppul.23165.
Jensen ME, Gibson PG, Collins CE, Wood LG. Airway and systemic inflammation in obese children with asthma. Eur Respir J. 2013;42(4):1012–9.
Simpson JL, Baines KJ, Ryan N, Gibson PG. Neutrophilic asthma is characterised by increased rhinosinusitis with sleep disturbance and GERD. Asian Pac J Allergy Immunol. 2014;32(1):66–74. Comprehensive evaluation of risk factors and clinical features of neutrophilic asthma.
Baraldo S, Turato G, Bazzan E, Ballarin A, Damin M, Balestro E, et al. Noneosinophilic asthma in children: relation with airway remodelling. Eur Respir J. 2011;38(3):575–83.
Green RH, Brightling CE, Woltmann G, et al. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax. 2002;57:875–9.
Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67(7):835–46.
Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13(3):249–56.
Agache IO. Endotype driven treatment of asthma. Curr Treat Options Allergy. 2014;1:198–212. Description of the endotype-driven therapeutic strategies in Th2 and non-Th2 asthma.
Agache I, Sugita K, Morita H, Akdis M, Akdis CA. Current treatment options in allergy. The complex type 2 endotype in allergy and asthma: from laboratory to bedside. Curr Allergy Asthma Rep. 2015, in press. The first paper to describe the concept of a complex endotype and to pinpoint the major subendotypes of Th2 asthma.
Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, et al. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol. 2007;102:221–30.
Al Heialy S, McGovern TK, Martin JG. Insights into asthmatic airway remodelling through murine models. Respirology. 2011;16:589–97.
McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–97.
Yang M, Kumar RK, Foster PS. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-gamma and TLR4/MyD88 pathways. J Immunol. 2009;182:5107–15.
Bullone M, Moran K, Lavoie-Lamoureux A, Lavoie JP. PI3K and MAPKs regulate neutrophil migration toward the airways in heaves. J Vet Intern Med. 2013;27(1):164–70.
Padoan E, Ferraresso S, Pegolo S, Castagnaro M, Barnini C, Bargelloni L. Real time RT-PCR analysis of inflammatory mediator expression in recurrent airway obstruction-affected horses. Vet Immunol Immunopathol. 2013;156(3–4):190–9.
Wood LG, Simpson JL, Hansbro P, Gibson PG. Potentially pathogenic bacteria cultured from the sputum of stable asthmatics are associated with increased 8-isoprostane and airway neutrophilia. Free Radic Res. 2010;44:146–54.
Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, et al. Extracellular vesicles, especially derived from gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin Exp Allergy. 2013;43(4):443–54.
Wallace J, D’Silva L, Brannan J, Hargreave FE, Kanaroglou P, Nair P. Association between proximity to major roads and sputum cell counts. Can Respir J. 2011;18(1):13–8.
Simpson JL, Grissell TV, Douwes J, Scott RJ, Boyle MJ, Gibson PG. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax. 2007;62:211–8.
Baines KJ, Simpson JL, Wood LG, Scott RJ, Fibbens NL, Powell H, et al. Sputum gene expression signature of 6 biomarkers discriminates asthma inflammatory phenotypes. J Allergy Clin Immunol. 2014;133(4):997–1007. Very elegant demonstration of the discriminatory capacity between eosinophilic and non-eosinophilic asthma of the six gene signature.
Dejager L, Dendoncker K, Eggermont M, Souffriau J, Van Hauwermeiren F, Willart M, et al. Neutralizing TNFα restores glucocorticoid sensitivity in a mouse model of neutrophilic airway inflammation. Mucosal Immunol. 2015. doi:10.1038/mi.2015.12.
Mikami Y, Matsuzaki H, Horie M, Noguchi S, Jo T, Narumoto O, et al. Lymphotoxin β receptor signaling induces IL-8 production in human bronchial epithelial cells. PLoS One. 2014;9(12), e114791.
Baines KJ, Simpson JL, Bowden NA, Scott RJ, Gibson PG. Differential gene expression and cytokine production from neutrophils in asthma phenotypes. Eur Respir J. 2010;35(3):522–31.
Gao P, Gibson PG, Baines KJ, Yang IA, Upham JW, Reynolds PN, et al. Anti-inflammatory deficiencies in neutrophilic asthma: reduced galectin-3 and IL-1RA/IL-1ß. Respir Res. 2015;16(1):5.
Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15(10):938–46. Description of the interaction between a newly described phenotype of neutrophils (N2) and macrophages in the context of a type 2 immune response in the lungs of nematode infected animals.
Simpson JL, Gibson PG, Yang IA, Upham J, James A, Reynolds PN, et al. Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin Exp Allergy. 2013;43:29–35.
Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Resp Crit Care Med. 2005;172:559–65.
Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol. 2009;123:986–94.
Yu S, Kim HY, Chang Y-J, DeKruyff RH, Umetsu DT. Innate lymphoid cells and asthma. J Allergy Clin Immunol. 2014;133:943–50.
Kim HY, Lee HJ, Chang Y-J, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61.
Mease PJ. Inhibition of interleukin-17, interleukin-23 and the TH17 cell pathway in the treatment of psoriatic arthritis and psoriasis. Curr Opin Rheumatol. 2015;27(2):127–33.
Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–30.
Zhao J, Lloyd CM, Noble A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance an contribute to airway remodeling. Mucosal Immunol. 2013;6:335–46.
Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135.
Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med. 2010;104:1131–7.
Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–8.
Barczyk A, Pierzchala W, Sozañska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–33.
Chien JW, Lin CY, Yang KD, Lin CH, Kao JK, Tsai YG. Increased IL-17A secreting CD41 T cells, serum IL-17 levels and exhaled nitric oxide are correlated with childhood asthma severity. Clin Exp Allergy. 2013;43:1018–26.
Raedler D, Ballenberger N, Klucker E, Böck A, Otto R, Prazeres da Costa O, et al. Identification of novel immune phenotypes for allergic and non-allergic childhood asthma. J Allergy Clin Immunol. 2015;135(1):81–91.
Gupta A, Dimeloe S, Richards DF, Chambers ES, Black C, Urry Z, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69:508–15.
Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125(1):222–30.
Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134:1175–86. First study proving the existence of dual-positive Th2/Th17 cells in the airways and their association with asthma severity.
Nakajima S, Kitoh A, Egawa G, Natsuaki Y, Nakamizo S, Moniaga CS, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol. 2014;134(8):2122–30.
Ponichtera HE, Shainheit MG, Liu BC, Raychowdhury R, Larkin BM, Russo JM, et al. CD209a expression on dendritic cells is critical for the development of pathogenic Th17 cell responses in murine schistosomiasis. J Immunol. 2014;192:4655–65.
Sutherland TE, Logan N, Rückerl D, Humbles AA, Allan SM, Papayannopoulos V, et al. Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol. 2014;15(12):1116–25.
Mizutani N, Nabe T, Yoshino S. IL-17A promotes the exacerbation of IL-33-induced airway hyperresponsiveness by enhancing neutrophilic inflammation via CXCR2 signaling in mice. J Immunol. 2014;192(4):1372–84.
McAlees JW, Whitehead GS, Harley IT, Cappelletti M, Rewerts CL, Holdcroft AM, et al. Distinct Tlr4-expressing cell compartments control neutrophilic and eosinophilic airway inflammation. Mucosal Immunol. 2014. doi:10.1038/mi.2014.117.
Furukawa T, Sakagami T, Koya T, Hasegawa T, Kawakami H, Kimura Y, et al. Characteristics of eosinophilic and non-eosinophilic asthma during treatment with inhaled corticosteroids. J Asthma. 2014;11:1–6.
Stein J, Maxeiner JH, Montermann E, Höhn Y, Raker V, Taube C, et al. Non-eosinophilic airway hyper-reactivity in mice, induced by IFN-γ producing CD4(+) and CD8(+) lung T cells, is responsive to steroid treatment. Scand J Immunol. 2014;80(5):327–38.
Li JJ, Tay HL, Maltby S, Xiang Y, Eyers F, Hatchwell L, et al. MicroRNA-9 regulates steroid-resistant airway hyperresponsiveness by reducing protein phosphatase 2A activity. J Allergy Clin Immunol. 2015. doi:10.1016/j.jaci.2014.11.044.
Clifford RL, Patel JK, John AE, Tatler AL, Mazengarb L, Brightling CE, et al. CXCL8 Histone H3 acetylation is dysfunctional in airway smooth muscle in asthma: regulation by BET. Am J Physiol Lung Cell Mol Physiol. 2015. doi:10.1152/ajplung.00021.2015.
Tsai CL, Lee WY, Hanania NA, Camargo Jr CA. Age-related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252–8.
Brooks CR, Gibson PG, Douwes J, Van Dalen CJ, Simpson JL. Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics. Respirology. 2013;18(5):857–65. First study showing a relation between increasing age and increasing sputum neutrophils, both in healthy and in asthmatics.
Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68(4):322–9.
Tong X, Guo T, Liu S, Peng S, Yan Z, Yang X, et al. Macrolide antibiotics for treatment of asthma in adults: A meta-analysis of 18 randomized controlled clinical studies. Pulm Pharmacol Ther. 2015;31:99–108.
Slater M, Rivett DW, Williams L, Martin M, Harrison T, Sayers I, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2014;69(7):673–4. This is the first study to examine longitudinal changes in airway microbiota following antibiotic treatment in asthma.
Essilfie AT, Horvat JC, Kim RY, Mayall JR, Pinkerton JW, Beckett EL, et al. Macrolide therapy suppresses key features of experimental steroid-sensitive and steroid-insensitive asthma. Thorax. 2015. doi:10.1136/thoraxjnl-2014-206067.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:1294–302. First large clinical trial to evaluate the efficacy and safety of anti-IL-17 targeted intervention in asthma and delineating a possible responder subgroup.
Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O’Byrne PM, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42(7):1097–103.
Austin RP, Bennion C, Bonnert RV, Cheema L, Cook AR, Cox RJ, et al. Discovery and evaluation of a novel monocyclic series of CXCR2 antagonists. Bioorg Med Chem Lett. 2015;25(7):1616–20.
Compliance with Ethics Guidelines
Conflict of Interest
Ioana Agache declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Asthma
Rights and permissions
About this article
Cite this article
Agache, I. Non-eosinophilic Asthma Endotypes. Curr Treat Options Allergy 2, 257–267 (2015). https://doi.org/10.1007/s40521-015-0052-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-015-0052-2