Abstract
Individuals with chronic obstructive pulmonary disease (COPD) are prone to malnutrition and sarcopenia as a result of nutritional deficiencies and increased energy metabolism. However, the effects of nutrient supplements (NS) on treating sarcopenia in patients with COPD are not well established from systematic evidence. This meta-analysis examined the effect of NS on sarcopenia in patients with COPD. A systematic search of multiple databases was conducted, and 29 randomized controlled trials involving 1625 participants (age, mean [SD] = 67.9 [7.8] years) were analyzed. NS demonstrated significant improvements in body weight (MD,1.33 kg; 95% CI, 0.60, 2.05 kg; P = 0.0003; I2 = 87%), fat-free mass index (MD, 0.74 kg/m2; 95% CI, 0.21, 1.27 kg/m2; P = 0.007; I2 = 75%), and 6-min walk test (MD, 19.43 m; 95% CI, 4.91, 33.94 m; P = 0.009; I2 = 81%) compared with control. However, NS had nonsignificant effects on handgrip strength (SMD, 0.36; 95% CI, − 0.15, 0.88; P = 0.16; I2 = 87%) and quadriceps muscle strength (SMD, 0.11; 95% CI, − 0.06, 0.27; P = 0.20; I2 = 25%) compared with the control. In conclusion, NS may be an effective treatment for improving body composition and physical performance in COPD. Future studies should explore the effects of intervention durations, specific NS types, or combined training in patients with COPD and sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by repeated exposure to noxious stimuli, such as tobacco smoke and air pollutants [1]. COPD is a major cause of morbidity, mortality, and healthcare use worldwide, particularly as life expectancy increases and ambient air pollution worsens because of rapid economic development [2]. According to the Global Burden of Diseases Study, approximately 3.3 million deaths and 74.4 million disability-adjusted life years were attributed to COPD in 2019 [3].
Muscle atrophy (i.e., secondary sarcopenia) is a result of physiological changes specific to COPD [4]. Approximately 27.5% of patients with COPD suffer from sarcopenia, particularly those at Global Initiative for COPD (GOLD) stages III–IV [5]. Patients with COPD and sarcopenia may have a worse prognosis because of impaired respiratory and peripheral muscle function, aggravating dyspnea, and reduced exercise capacity [6].
With several effective treatments for COPD, effective management strategies can alleviate the disease burden and improve patients’ quality of life [3, 7]. Currently, nutritional strategies are one of the cornerstones for managing COPD-related sarcopenia to reduce the effects of COPD on muscles and regulate the metabolism of muscle protein in patients with COPD [8, 9].
In a series of meta-analyses, nutritional supplements, with or without exercise, reduced the risk of sarcopenia in adults [10,11,12]. Nutritional support for muscle mass or muscle function in patients with COPD has been systematically reviewed; however, the pooled analysis of outcome measurements in sarcopenia is incomplete [13, 14]. A review showed improved fat-free mass (FFM), muscle strength, and physical performance in patients with COPD; however, the findings were narratively recapped [15].
Nutrient supplements (NS) refer to a type of health products containing one or several natural plant or animal nutrients or synthetic nutrients [16]. NS are used to compensate for nutrient deficiencies in people with COPD; however, their effects remain unclear. In addition, patients’ intervention duration, rehabilitation duration, or NS type may have affected their outcomes. However, evidence on the quantitative effects of NS interventions is limited. Thus, this systematic review and meta-analysis examined the effects of NS on the treatment of sarcopenia in patients with COPD.
Methods
Search strategy
We performed the review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement and registered it in PROSPERO International prospective register of systematic reviews (ID: CRD42022337646) [17].
The following electronic databases were searched from inception until July 5, 2022: PubMed, Embase, the Cochrane Library, Web of Science, Ovid, Scopus, and the International Clinical Trials Registry Platform. Publication dates and geographical area were not restricted. Keywords in accordance with PICOS structure, as follows: population: COPD patients; intervention: NS; comparison: no NS; outcome: sarcopenia; study design: randomized controlled trials (RCTs). The detailed search strategy was showed in Table S1.
Eligibility criteria
Articles included in the study were: (i) original studies written in English; (ii) designed as RCTs; (iii) examining subjects with COPD; (iv) using NS as an intervention (including those concurrently using or comparing it with standard diet and/or dietary counseling); and (v) reporting measurements of at least one of the following: muscle mass, muscle strength or physical performance.
We excluded articles when they failed to meet the inclusion criteria or met one of the following exclusion criteria: (i) the intervention was enteral tube feeding, parenteral nutrition, dietary counseling alone, or snacks; (ii) the nutritional intervention was combined with a pharmaceutical intervention (e.g., with anabolic steroids); (iii) the average duration of the intervention was less than two weeks; (iv) the study design was an animal trial, observational trial, case report, opinion letter, literature review, systematic review, or meta-analysis; (v) the study had a high risk of overall bias; and (vi) data extraction was not possible.
Article selection
All articles were imported into EndNote software, and duplicates were removed. Two researchers independently screened titles and abstracts and read all potentially eligible publications. Any uncertainties in the selection process were discussed and settled with a third investigator.
Data extraction and quality assessment
For each study, a researcher extracted data using a pre-determined data form that included the first author’s last name, publication data, country, participant characteristics (age, gender, and health status), intervention type and dose, outcome measurements, and other baseline information. Data extraction was independently verified by other authors.
To assess the differences between the experimental and control groups, changes in mean and standard deviation (SD) were employed as summary statistics. GetData Graph Digitizer version 2.24 was utilized for extracting values from graphs when numerical data were not directly available. For studies that did not report changes in SD, attempts were made to contact the corresponding authors for additional information. Following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, in cases where these efforts were unsuccessful, correlation coefficients (corr) were calculated to estimate the changes in SD [18]:
The following formula was then applied to calculate the changes in SD:
Using version 2 of the Cochrane risk-of-bias tool (RoB 2) for RCTs, two researchers independently assessed the quality of included studies. The RoB 2 covers five areas of bias: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. A risk-of-bias judgment were made for each domain and overall as low, some concerns, or high [19].
Data synthesis and analysis
An analysis of pre- and post-treatment changes for intervention and control groups were performed to examine the efficacy of NS on sarcopenia, generating pooled values in form of mean differences (MDs) and 95% confidence intervals (CIs). When the units of outcome measurements were inconsistent, standardized mean differences (SMDs) were used. Based on I2 statistics, the heterogeneity of outcomes between studies was determined. I2 ≤ 50% was considered low heterogeneity, and the data were pooled using a fixed-effect model; while I2 > 50%, the data were pooled using a random-effect model. The results of this meta-analysis were visualized using forest plots.
Moreover, we conducted multiple sensitivity analyses by removing one study at a time to determine the effect of each included study on the pooled effect. We conducted subgroup analyses based on intervention periods, pulmonary rehabilitation presence or absence, and NS types. Review Manager (RevMan 5.3) was used for all statistical analyses.
Results
Search and study characteristics
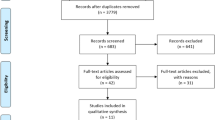
The selection process of studies for the meta-analysis is shown in Fig. 1. In total, 1903 articles were identified through searches, of which 29 were included in the final analysis [9, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47]. The overall mean ± SD age of 1625 participants in 29 studies was 67.9 ± 7.8 years. Moreover, 817 participants received NS, and 808 received usual care or placebo.
The meta-analysis includes 29 studies, summarized in Table 1. There were three studies that had a gender-specific design for COPD patients who were male [23, 30, 36]; over 70% of the COPD patients in eight studies were male [20, 25, 31, 34, 37, 39, 41, 47]; two studies did not report the number of men and women [33, 35]. Except for two studies on admission, almost all participants with COPD were stable [21, 41]. Moreover, participants in ten studies were malnourished, manifested as low body mass index (BMI) criteria, low muscle mass, or recent involuntary weight loss [21, 22, 26,27,28, 34, 37, 38, 41, 47].
The intervention periods ranged from 4 to 24 weeks. NS interventions were compared with usual care or placebo supplements in all studies. Nutrition supplements were diverse among included studies. Six studies were focused on providing energy-type NS, with prescribed energy ranging from 90 to 960 kcal/d [22, 31, 33, 34, 46, 47]. Six studies provided essential amino acids (EAAs) either alone [38, 41] or EAA-enriched energy-type NS [9, 27, 32, 37]. Three studies supplemented whey protein [23, 26, 40] and one studies supplemented β-hydroxy β-methylbutyrate [21]. In five studies, creatine was consumed either alone [42, 43, 45] or in combination with coenzyme QTer® [25, 35]. There studies used vitamin D3 alone [28, 36, 39], and there studies used vitamin B12 [29], polyunsaturated fatty acids [44], or magnesium citrate as supplements [20], respectively. In two studies, nutritional antioxidant supplements were used [24, 30]. In all but 1 trial the NS was given daily; in the 1 study it was consumed 100,000 IU of vitamin D per month [39]. A total of 16 studies included pulmonary rehabilitation throughout their study periods, ranging from 2 to 7 sessions per week [9, 22, 24, 27, 29, 32, 34, 37, 39,40,41,42,43,44,45,46].
Quality assessment
Figure 2 summarized the risk of bias for the included studies, while Figure S1 listed the risk of bias for each study. Eight studies raised some concerns about the randomization process due to a lack of information regarding randomization and concealment procedures [9, 33, 35, 40, 41, 44, 45, 47]. One study had some concerns arising from absence of a concealment procedure and inadequate follow-up [30]. One study had some concerns because there was no concealment procedure and no placebo preparation, which compromised the blinding of the control group [32]. One study had some concerns arising from absence of information on dropout rate [38].
Effects of NS on body weight (BW) and BMI
Meta-analysis of mean differences in BW included eighteen studies with 410 NS participants and 418 control participants. In Fig. 3A, NS showed a positive effect on improving BW (MD: 1.33 kg; 95% CI: 0.60, 2.05 kg; P = 0.0003; I2 = 87%). Analyzing sensitivity revealed similar results.
BMI was compared between NS groups and control groups in fourteen studies. As shown in Fig. 3B, BMI was close to statistical significance with a small effect size (MD: 0.41 kg/m2; 95% CI: -0.01, 0.83 kg/m2; P = 0.06; I2 = 83%). However, sensitivity analyses found no heterogeneity among studies after the exclusion of Dal Negro 2012 study [38] (I2 = 0%), with a significant increase in BMI (MD: 0.27 kg/m2; 95% CI: 0.10, 0.43 kg/m2; P = 0.002; I2 = 0%) in favor of NS.
Effects of NS on muscle mass
There were seventeen RCTs that provided data to reflect between-group difference in muscle mass, measured by FFM, fat-free mass index (FFMI), arm circumference (AC), calf circumference (CC), and mid-thigh cross-sectional area (mid-thigh CSA) (Fig. 4). The meta-analysis found a significant increase in FFMI (MD: 0.74 kg/m2; 95% CI: 0.21, 1.27 kg/m2; P = 0.007; I2 = 75%) following NS treatment, but no significant effect on FFM (MD: 0.42 kg; 95% CI: − 0.24, 1.08 kg; P = 0.21; I2 = 54%), AC (MD: 0.01 cm; 95% CI: − 0.37, 0.39 cm; P = 0.96; I2 = 0%), CC (MD: 0.09 cm; 95% CI: − 0.58, 0.76 cm; P = 0.79; I2 = 0%), and mid-thigh CSA (MD: 0.09 cm2; 95% CI: − 3.96, 4.15 cm2; P = 0.96; I2 = 0%) following NS treatment compared to the control group.
In the sensitivity analyses, the heterogeneity was reduced after removing Marinari 2013 results [35] from the pooled estimate of FFMI, but the statistical significance was not changed. Other outcomes of muscle mass were not affected by sensitivity analyses.
Effects of NS on muscle strength
The effects of NS on the muscle strength of people with COPD were determined by measuring handgrip strength (HGS) and quadriceps muscle strength (QMS). There were non-significant effects on HGS and QMS with SMDs of 0.36 (95% CI: − 0.15, 0.88; P = 0.16; I2 = 87%) and 0.11 (95% CI: − 0.06, 0.27; P = 0.20; I2 = 25%), respectively, in meta-analyses (Fig. 5).
The exclusion of Dal Negro 2012 study [38] resulted in a decrease in heterogeneity among studies (I2 = 3%), but the statistical significance was not changed in HGS (SMD: 0.17; 95% CI: -0.02, 0.35; P = 0.07; I2 = 3%). In addition, no study changed QMS outcomes based on sensitivity analyses.
Effects of NS on physical performance
Several tests were used to estimate the treatment effects of NS on physical performance: 6-min walk test (6-MWT), physical activity level (PAL), incremental shuttle walk test (ISWT), endurance shuttle walk test (ESWT), short physical performance battery (SPPB), five-repetition sit-to-stand test (STS5), and cycle endurance time (CET) (Fig. 6 and Fig. S2). NS showed significant benefits to 6-MWT (MD: 19.43 m; 95% CI: 4.91, 33.94 m; P = 0.009; I2 = 81%) (Fig. 6). Furthermore, no study changed physical performance outcomes based on sensitivity analyses.
Subgroup analyses
Stratified analyses showed that intervention durations appeared to have no effect on the role of NS in improving sarcopenia (Figs. S3–S9). There was no significant change in the effects of NS on sarcopenia regardless of whether NS was combined with pulmonary rehabilitation (Figs. S10–S16). Contrary to this, 6-MWT appeared to have a benefit in the absence of pulmonary rehabilitation, although it was not statistically significant (P = 0.08; Fig. S16). Subgroup analyses comparing the types of NS revealed no significant differences (Figs. S17–S21).
Discussion
In this meta-analysis, NS was evaluated for its efficacy in treating patients with COPD predisposed to sarcopenia. Compared with control (usual care or placebo supplements), NS positively affected BW, FFMI, 6-MWT, PAL, and STS5 in patients with COPD. In contrast, NS did not improve muscle strength (HGS and QMS) in patients with COPD. In addition, insufficient studies were included in the subgroup analyses to derive the effect of intervention durations, specific NS types, or combined training on the role of NS in improving sarcopenia.
Systematic reviews have found inconsistent results regarding nutritional support in patients with COPD. In earlier studies, nutritional support failed to demonstrate benefits for patients with COPD [48,49,50,51]. Although muscle strength was not improved by NS, the overall effects were consistent with the improvements in clinically relevant outcomes observed in recent meta-analysis studies of nutritional interventions [13, 14, 52, 53]. According to these findings, nutritional support is crucial in the treatment of patients with COPD.
Patients with COPD may respond satisfactorily to NS, resulting in augmentation of BW and muscle mass during the intervention. Based on an epidemiologic study involving 1898 participants, Vestbo et al. reported an increased mortality rate among patients with COPD having low BMI or FFMI [54]. Reduced nutrient intake contributed to weight loss or muscle loss in patients with COPD [55]. Collins et al. reported that NS can improve the nutritional status of patients and overcome energy imbalance [53]. No response to NS was noted in AC, CC, and midthigh CAS, suggesting that patients with COPD may have a balanced distribution of increased FFM throughout their bodies.
HGS is used extensively in studies focusing on sarcopenia because of its practicality and low cost in the clinical setting [56]. The HGS results of this review differed from those of previous systematic reviews regarding NS for patients with COPD. According to a previous study, the NS group had improved HGS compared with the control group when dietary counseling and enteral nutrition were included as interventions [13]. In addition, a positive effect of nutritional support was found in the pooled estimates of HGS expressed as percentage changes [14, 53]. In a 4-year prospective study of 3018 older adults, the loss of HGS rapidly outpaced the loss of muscle mass, suggesting that the positive effects of NS on HGS may require longer-term intervention in patients with COPD [57].
Patients with COPD showed significant improvements in 6-MWT, PAL, and STS5 levels after NS intervention. NS was effective in treating patients with COPD; however, previous meta-analyses excluded physical performance as a measurement outcome [13, 14, 52, 53]. In people with chronic respiratory disease, 6-MWT, ISWT, and ESWT are commonly used to assess exercise capacity [58]. An association was found between 6-MWT and mortality, and ISWT was a predictor of survival. However, fewer studies have examined ESWT in patients with COPD [58]. The minimal important differences for 6-MWT, ISWT, and ESWT were 30 m, 47.5 m, and 45–85 s, respectively [34, 58]. 6-MWT significantly improved after NS; however, ISWT and ESWT require further studies. Patients’ daily habits have a major effect on their PAL, which represents their average number of steps per day [38]. Therefore, this result should be interpreted with caution because of its limited accuracy. In addition, the SPPB or STS5 results were based on a meta-analysis of only one study; therefore, well-designed RCTs are needed to examine the effects of NS on SPPB or STS5. In patients with COPD, CET is used to assess exercise tolerance and is not interchangeable with 6-MWT [59]. Interestingly, patients with rehabilitation training reported no significant improvement in CET after 3 times/week endurance and resistance training [27, 29, 40]; however, patients with NS without rehabilitation training showed the greatest improvement [29]. These findings suggest that posttraining fatigue may affect outcome measurements or that dietary supplements fail to counteract exercise exertion.
In the subgroup analysis, no difference was found between long-term (≥ 12 weeks) and short-term (< 12 weeks) intervention in improving sarcopenia in patients with COPD. Surprisingly, NS combined with pulmonary rehabilitation reduced 6-MWT more than presented a greater benefit, suggesting that posttraining fatigue may affect outcome measurements or dietary supplements fail to counteract exercise exertion. The combined intervention also reduced mobility in malnourished older adults compared with NS alone [60]. Ongoing calorie supplementation through NS significantly improves the BW of malnourished patients with COPD and their quality of life [61]. On the basis of our findings, the study failed to present a difference in the role of NS providing energy nutrients versus NS providing nonenergy nutrients for treating sarcopenia in patients with COPD.
Patients with COPD have different degrees of muscle atrophy (i.e., secondary sarcopenia) because of advanced age, less physical activity, hypoxemia, systemic inflammation, reduced nutritional intake, and glucocorticoids [55, 62]. Sarcopenia is a key extrapulmonary feature of COPD and is characterized by reductions in muscle quality and quantity [4, 5]. Patients with COPD and sarcopenia have impaired physical function, increased degrees of dyspnea, poor prognosis, and a high risk of exacerbation or death [5, 6]. It can significantly improve the health-related quality of life of patients with COPD by reducing the risk of sarcopenia. Malnutrition is a correctable risk factor for sarcopenia. An NS that targets nutrient provision can correct a reduction in nutritional intake. Evidence containing nearly half of the low-to-moderate quality RCTs showed that NS could improve body composition and physical performance in patients with COPD. This study highlights the need for future high-quality RCTs that use standardized outcomes of sarcopenia when exploring the role of NS in treating sarcopenia.
Several limitations should be considered in this study. First, although the NS regimens were homogeneous among the included studies, some variations were noted among NS regimens; therefore, determining the effect of a specific type of NS on sarcopenia was difficult, and the clinical heterogeneity inevitably affected the results. Second, the subgroup analysis lacked statistical power. Fewer than three RCTs were included in subgroup analyses stratified by NS type, suggesting that such analyses were not sufficiently powerful to detect changes. Third, the severity of COPD could not be classified. Indeed, most of the included studies enrolled patients with moderate-to-severe COPD; however, the use of the criterion of FEV1 less than the percentage of predicted values prevented us from categorizing the whole sample. Finally, sensitivity analyses were conducted to rule out possible effects; however, heterogeneity related to race and measurement tools was not negligible, affecting the reliability of the evidence.
Conclusion
In this systematic review and meta-analysis, NS was associated with weight gain and increases in FFMI, 6-MWT, PAL, and STS5, whereas increases in muscle strength may require longer NS interventions. A subgroup analysis revealed a downward trend in 6-MWT following combined pulmonary rehabilitation, indicating a potential effect of posttraining fatigue or increased energy expenditure after exercise. The effect of NS types on sarcopenia was inconclusive because of the lack of trials. Further research on intervention durations, NS types, or NS combined training in populations with COPD is required to gain insight into their effects on sarcopenia. Based on the available evidence, NS is a feasible treatment option for COPD-related sarcopenia.
Data availability
The data used to support the findings of this study have been included in this article.
References
Jarhyan P, Hutchinson A, Khaw D et al (2022) Prevalence of chronic obstructive pulmonary disease and chronic bronchitis in eight countries: a systematic review and meta-analysis. Bull World Health Organ 100:216–230. https://doi.org/10.2471/BLT.21.286870
Christenson SA, Smith BM, Bafadhel M et al (2022) Chronic obstructive pulmonary disease. Lancet 399:2227–2242. https://doi.org/10.1016/S0140-6736(22)00470-6
Safiri S, Carson-Chahhoud K, Noori M et al (2022) Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: results from the Global Burden of Disease Study 2019. BMJ 378:e069679. https://doi.org/10.1136/bmj-2021-069679
Bauer J, Morley JE, Schols AMWJ et al (2019) Sarcopenia: a time for action An SCWD position paper. J Cachexia Sarcopenia Muscle 10:956–961. https://doi.org/10.1002/jcsm.12483
Sepúlveda-Loyola W, Osadnik C, Phu S et al (2020) Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 11:1164–1176. https://doi.org/10.1002/jcsm.12600
Wouters EF, Creutzberg EC, Schols AM (2002) Systemic effects in COPD. Chest 121:127S-130S. https://doi.org/10.1378/chest.121.5_suppl.127s
Ng-Blichfeldt JP, Gosens R, Dean C et al (2019) Regenerative pharmacology for COPD: breathing new life into old lungs. Thorax 74:890–897. https://doi.org/10.1136/thoraxjnl-2018-212630
van Bakel SIJ, Gosker HR, Langen RC et al (2021) Towards personalized management of sarcopenia in COPD. Int J Chron Obstruct Pulmon Dis 16:25–40. https://doi.org/10.2147/COPD.S280540
de Bisschop C, Caron F, Ingrand P et al (2021) Does branched-chain amino acid supplementation improve pulmonary rehabilitation effect in COPD? Respir Med 189:106642. https://doi.org/10.1016/j.rmed.2021.106642
Malafarina V, Uriz-Otano F, Iniesta R et al (2013) Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc 14:10–17. https://doi.org/10.1016/j.jamda.2012.08.001
Liao CD, Tsauo JY, Wu YT et al (2017) Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr 106:1078–1091. https://doi.org/10.3945/ajcn.116.143594
Hanach NI, McCullough F, Avery A (2019) The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr 10:59–69. https://doi.org/10.1093/advances/nmy065
Bernardes S, Eckert IC, Burgel CF et al (2022) Increased energy and/or protein intake improves anthropometry and muscle strength in COPD patients: a systematic review with meta-analysis on randomized controlled clinical trials. Br J Nutr. https://doi.org/10.1017/S0007114522000976
Collins PF, Elia M, Stratton RJ (2013) Nutritional support and functional capacity in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respirology 18:616–629. https://doi.org/10.1111/resp.12070
Huang WJ, Fan XX, Yang YH et al (2022) A review on the role of oral nutritional supplements in chronic obstructive pulmonary disease. J Nutr Health Aging 26:723–731. https://doi.org/10.1007/s12603-022-1822-8
Gong W, Liu A, Yao Y et al (2018) Nutrient supplement use among the Chinese Population: a cross-sectional study of the 2010–2012 China nutrition and health surveillance. Nutrients 10:1733. https://doi.org/10.3390/nu10111733
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JPT, Li T, Deeks JJ (2021) Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, et al (eds) Cochrane handbook for systematic reviews of interventions, 6.2rd edn, Cochrane, section-6–5–2–8 Online document. Available from www.training.cochrane.org/handbook. Accessed 25 Aug 2022
Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Zanforlini BM, Ceolin C, Trevisan C et al (2022) Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin Exp Res 34:167–174. https://doi.org/10.1007/s40520-021-01921-z
Deutz NE, Ziegler TR, Matheson EM et al (2021) Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin Nutr 40:1388–1395. https://doi.org/10.1016/j.clnu.2020.08.031
Aldhahir AM, Aldabayan YS, Alqahtani JS et al (2021) A double-blind randomised controlled trial of protein supplementation to enhance exercise capacity in COPD during pulmonary rehabilitation: a pilot study. ERJ Open Res 7:00077–02021. https://doi.org/10.1183/23120541.00077-2021
Ahmadi A, Eftekhari MH, Mazloom Z et al (2020) Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: a single-blind, randomized clinical trial. Respir Res 21:216. https://doi.org/10.1186/s12931-020-01466-1
Gouzi F, Maury J, Héraud N et al (2019) Additional effects of nutritional antioxidant supplementation on peripheral muscle during pulmonary rehabilitation in COPD patients: a randomized controlled trial. Oxid Med Cell Longev 2019:5496346. https://doi.org/10.1155/2019/5496346
De Benedetto F, Pastorelli R, Ferrario M et al (2018) Supplementation with Qter® and creatine improves functional performance in COPD patients on long term oxygen therapy. Respir Med 142:86–93. https://doi.org/10.1016/j.rmed.2018.08.002
Calder PC, Laviano A, Lonnqvist F et al (2018) Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. J Cachexia Sarcopenia Muscle 9:28–40. https://doi.org/10.1002/jcsm.12228
van de Bool C, Rutten EPA, van Helvoort A et al (2017) A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle 8:748–758. https://doi.org/10.1002/jcsm.12219
Rafiq R, Prins HJ, Boersma WG et al (2017) Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: a pilot trial. Int J Chron Obstruct Pulmon Dis 12:2583–2592. https://doi.org/10.2147/COPD.S132117
Paulin FV, Zagatto AM, Chiappa GR et al (2017) Addition of vitamin B12 to exercise training improves cycle ergometer endurance in advanced COPD patients: a randomized and controlled study. Respir Med 122:23–29. https://doi.org/10.1016/j.rmed.2016.11.015
Pirabbasi E, Shahar S, Manaf ZA et al (2016) Efficacy of ascorbic acid (vitamin C) and/n-acetylcysteine (NAC) supplementation on nutritional and antioxidant status of male chronic obstructive pulmonary disease (COPD) patients. J Nutr Sci Vitaminol (Tokyo) 62:54–61. https://doi.org/10.3177/jnsv.62.54
Khan NA, Kumar N, Daga MK (2016) Effect of dietary supplementation on body composition, pulmonary function and health-related quality of life in patients with stable COPD. Tanaffos 15:225–235 (PMID: 28469679)
Ahnfeldt-Mollerup P, Hey H, Johansen C et al (2015) The effect of protein supplementation on quality of life, physical function, and muscle strength in patients with chronic obstructive pulmonary disease. Eur J Phys Rehabil Med 51:447–456 (PMID: 25426541)
Raizada N, Daga MK, Kumar N et al (2014) Nutritional intervention in stable COPD patients and its effect on anthropometry, pulmonary function, and health-related quality of life (HRQL). J Indian Acad Clin Med 15:100–105 (PMID: 264553379)
Gurgun A, Deniz S, Argın M et al (2013) Effects of nutritional supplementation combined with conventional pulmonary rehabilitation in muscle-wasted chronic obstructive pulmonary disease: a prospective, randomized and controlled study. Respirology 18:495–500. https://doi.org/10.1111/resp.12019
Marinari S, Manigrasso MR, De Benedetto F (2013) Effects of nutraceutical diet integration, with coenzyme Q10 (Q-Ter multicomposite) and creatine, on dyspnea, exercise tolerance, and quality of life in COPD patients with chronic respiratory failure. Multidiscip Respir Med 8:40. https://doi.org/10.1186/2049-6958-8-40
Bjerk SM, Edgington BD, Rector TS et al (2013) Supplemental vitamin D and physical performance in COPD: a pilot randomized trial. Int J Chron Obstruct Pulmon Dis 8:97–104. https://doi.org/10.2147/COPD.S40885
Sugawara K, Takahashi H, Kashiwagura T et al (2012) Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir Med 106:1526–1534. https://doi.org/10.1016/j.rmed.2012.07.001
Dal Negro RW, Testa A, Aquilani R et al (2012) Essential amino acid supplementation in patients with severe COPD: a step towards home rehabilitation. Monaldi Arch Chest Dis 77:67–75. https://doi.org/10.4081/monaldi.2012.154
Hornikx M, Van Remoortel H, Lehouck A et al (2012) Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res 13:84. https://doi.org/10.1186/1465-9921-13-84
Laviolette L, Lands LC, Dauletbaev N et al (2010) Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food 13:589–598. https://doi.org/10.1089/jmf.2009.0142
Baldi S, Aquilani R, Pinna GD et al (2010) Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J Chron Obstruct Pulmon Dis 5:29–39. https://doi.org/10.2147/copd.s7739
Deacon SJ, Vincent EE, Greenhaff PL et al (2008) Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 178:233–239. https://doi.org/10.1164/rccm.200710-1508OC
Faager G, Söderlund K, Sköld CM et al (2006) Creatine supplementation and physical training in patients with COPD: a double blind, placebo-controlled study. Int J Chron Obstruct Pulmon Dis 1:445–453. https://doi.org/10.2147/copd.2006.1.4.445
Broekhuizen R, Wouters EF, Creutzberg EC et al (2005) Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax 60:376–382. https://doi.org/10.1136/thx.2004.030858
Fuld JP, Kilduff LP, Neder JA et al (2005) Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 60:531–537. https://doi.org/10.1136/thx.2004.030452
Steiner MC, Barton RL, Singh SJ et al (2003) Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 58:745–751. https://doi.org/10.1136/thorax.58.9.745
Lewis MI, Belman MJ, Dorr-Uyemura L (1987) Nutritional supplementation in ambulatory patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 135:1062–1068. https://doi.org/10.1164/arrd.1987.135.5.1062
Ferreira IM, Brooks D, Lacasse Y et al (2000) Nutritional support for individuals with COPD: a meta-analysis. Chest 117:672–678. https://doi.org/10.1378/chest.117.3.672
Ferreira IM, Brooks D, Lacasse Y et al (2002) Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002:CD000998. https://doi.org/10.1002/14651858.CD000998
Ferreira IM, Brooks D, Lacasse Y et al (2005) Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005:CD000998. https://doi.org/10.1002/14651858.CD000998.pub2
Al-Ghimlas F, Todd DC (2010) Creatine supplementation for patients with COPD receiving pulmonary rehabilitation: a systematic review and meta-analysis. Respirology 15:785–795. https://doi.org/10.1111/j.1440-1843.2010.01770.x
Ferreira IM, Brooks D, Lacasse Y et al (2012) Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012:CD000998. https://doi.org/10.1002/14651858.CD000998.pub3
Collins PF, Stratton RJ, Elia M (2012) Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr 95:1385–1395. https://doi.org/10.3945/ajcn.111.023499
Vestbo J, Prescott E, Almdal T et al (2006) Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med 173:79–83. https://doi.org/10.1164/rccm.200506-969OC
Langen RC, Gosker HR, Remels AH et al (2013) Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol 45:2245–2256. https://doi.org/10.1016/j.biocel.2013.06.015
Chen LK, Woo J, Assantachai P et al (2020) Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21:300-307.e2. https://doi.org/10.1016/j.jamda.2019.12.012
Auyeung TW, Lee SW, Leung J et al (2014) Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int 14:76–84. https://doi.org/10.1111/ggi.12213
Holland AE, Spruit MA, Troosters T et al (2014) An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 44:1428–1446. https://doi.org/10.1183/09031936.00150314
Andrianopoulos V, Wagers SS, Groenen MT et al (2014) Characteristics and determinants of endurance cycle ergometry and six-minute walk distance in patients with COPD. BMC Pulm Med 14:97. https://doi.org/10.1186/1471-2466-14-97
Wright J, Baldwin C (2018) Oral nutritional support with or without exercise in the management of malnutrition in nutritionally vulnerable older people: a systematic review and meta-analysis. Clin Nutr 37:1879–1891. https://doi.org/10.1016/j.clnu.2017.09.004
Hsieh MJ, Yang TM, Tsai YH (2016) Nutritional supplementation in patients with chronic obstructive pulmonary disease. J Formos Med Assoc 115:595–601. https://doi.org/10.1016/j.jfma.2015.10.008
Bird JK, Troesch B, Warnke I et al (2021) The effect of long chain omega-3 polyunsaturated fatty acids on muscle mass and function in sarcopenia: a scoping systematic review and meta-analysis. Clin Nutr ESPEN 46:73–86. https://doi.org/10.1016/j.clnesp.2021.10.011
Funding
Academic funding from the Second Affiliated Hospital of Fujian Medical University (Serial No. BS201902) and the Fujian Province Science and Technology Project, China, under contract No. 2021J01267.
Author information
Authors and Affiliations
Contributions
Conceptualization: C.Y.K. and W.J.H.; Methodology: C.Y.K. and W.J.H.; Software: W.J.H.; Formal Analysis: W.J.H.; Investigation: W.J.H.; Resources, C.Y.K. and W.J.H. Data Curation, W.J.H.; Writing – Original Draft Preparation, C.Y.K. and W.J.H.; Writing – Review & Editing: C.Y.K. and W.J.H.; Visualization: C.Y.K. and W.J.H.; Supervision: C.Y.K.; Project Administration: C.Y.K.; Funding Acquisition: C.Y.K. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Ethical approval
The study was approved by the Ethical Committee of the Second Affiliated Hospital of Fujian Medical University (Ethics Review No. 2021-31).
Statement of human and animal rights
This study does not involve any human or animal testing.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, WJ., Ko, CY. Systematic review and meta-analysis of nutrient supplements for treating sarcopenia in people with chronic obstructive pulmonary disease. Aging Clin Exp Res 36, 69 (2024). https://doi.org/10.1007/s40520-024-02722-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-024-02722-w