Abstract
Background
Estimating the risks and impacts of COVID-19 for different health groups at the population level is essential for orienting public health measures. Adopting a population-based approach, we conducted a systematic review to explore: (1) the etiological role of multimorbidity and frailty in developing SARS-CoV-2 infection and COVID-19-related short-term outcomes; and (2) the prognostic role of multimorbidity and frailty in developing short- and long-term outcomes. This review presents the state of the evidence in the early years of the pandemic. It was conducted within the European Union Horizon 2020 program (No: 101018317); Prospero registration: CRD42021249444.
Methods
PubMed, Embase, World Health Organisation COVID-19 Global literature on coronavirus disease, and PsycINFO were searched between January 2020 and 7 April 2021 for multimorbidity and 1 February 2022 for frailty. Quantitative peer-reviewed studies published in English with population-representative samples and validated multimorbidity and frailty tools were considered.
Results
Overall, 9,701 records were screened by title/abstract and 267 with full text. Finally, 14 studies were retained for multimorbidity (etiological role, n = 2; prognostic, n = 13) and 5 for frailty (etiological role, n = 2; prognostic, n = 4). Only short-term outcomes, mainly mortality, were identified. An elevated likelihood of poorer outcomes was associated with an increasing number of diseases, a higher Charlson Comorbidity Index, different disease combinations, and an increasing frailty level.
Discussion
Future studies, which include the effects of recent virus variants, repeated exposure and vaccination, will be useful for comparing the possible evolution of the associations observed in the earlier waves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the start of the COVID-19 pandemic in 2019, understanding the impact of SARS-CoV-2 infection on population health has become more coherent. Much evidence has emerged, among others, for different age groups [1,2,3], patient groups such as those with immunodeficiency or kidney transplant [4, 5] or population groups such as health care workers or pregnant women [6, 7] or on the specific effects of COVID-19 such as mental health consequences, though predominantly among non-infected individuals [8, 9].
Advanced age is repeatedly mentioned as an independent risk factor for death and other adverse outcomes among infected individuals [10, 11]. Findings from China in the early period of the pandemic showed an increase in the COVID-19-related case fatality ratio with age, of e.g., 0.4% or less in 40-year olds or younger, 1.3% among individuals in their 50s, 3.6% among individuals in their 60s, 8% among 70-year olds, and 14.8% in those aged 80 years or older [12]. Furthermore, older age is likely to be accompanied by one or multiple chronic diseases, commonly called multimorbidity [13], or the accumulation of deficits in different body systems, known as frailty [14]. In fact, 55 to 98% individuals over the age of 60 live with multiple chronic conditions [15], while multidimensional frailty prevalence ranges from 13.3% in population-based settings to 51.5% in nursing homes [16]. Moreover, it is worth recalling that the number of elderly people have been increasing worldwide for decades. The European Union, for example, had 90.5 million citizens aged 65 years and over in 2019 with projections of 129.8 million by 2050; the age group of 85 and older is growing at the fastest pace, with a projected increase of approximately 113.9% by 2050 [17]. This demographic change is observed globally, thus highlighting the associated challenges such as multimorbidity and frailty.
The risks of poor COVID-19-related outcomes have been well documented in patients with single chronic conditions. Studies have underlined hypertension, diabetes, cardiovascular diseases and chronic pulmonary or kidney diseases as important factors contributing to the increased in-hospital case fatality rate [18]. However, despite growing challenges associated with disease accumulation in rapidly expanding ageing populations [19], exploring the interplay between different numbers and combinations of health conditions and their impact on COVID-19-related outcomes has not yet been sufficiently explored [18].
Both multimorbidity and frailty may interfere with the physiological processes of individuals, thus making them more susceptible to infection or adverse outcomes in COVID-19 illness. Evidence about their role in the COVID-19 pandemic identified increased mortality risks and intensive care unit (ICU) admissions in patients with frailty and multimorbidity [20, 21]. However, these findings are primarily based on hospital settings, which precludes the generalisation of the strength of these associations to the general population. Assessing and stratifying these risks at the population level is thus essential for informing public health decision-making and actions [22].
Using a population-based approach, we conducted a systematic literature review with two objectives: (1) to evaluate the etiological role of multimorbidity and frailty in developing SARS-CoV-2 infection and COVID-19-related short-term outcomes; and (2) to investigate the prognostic role of multimorbidity and frailty in developing short- and long-term COVID-19 outcomes. This paper reports all the outcomes identified in the literature meeting all the study criteria.
This study was part of a broader analytical work funded by the European Union’s (EU) Horizon 2020 research and innovation program under the grant agreement no. 101018317, which considered both biomedical and socioeconomic factors [23, 24]. This paper only focuses on biomedical factors, namely multimorbidity and frailty.
Methods
The study was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 [25]. The protocol was registered in the PROSPERO registry for systematic review protocols (no. CRD42021249444) and previously described [23]. Thus, only the main methodological points are outlined below.
Study design and search strategy
We applied a population-based approach and considered only studies conducted on well-defined populations of individuals residing in a defined geographic region in a given time period [26]. The analysis could include all individuals or a random population sample, the setting could be community- and/or hospital-based and the design could be based on case–control, cross-sectional or prospective and retrospective cohort studies. The key point is that the individuals included in the studies are representative of all individuals in the well-defined population [27].
All types of observational studies with comparative groups were eligible, either etiological: considering risk factors, believed to be related to the probability of an individual of the population developing SARS-CoV-2 infection or COVID-19-related health event; or prognostic: considering prognostic factors, believed to be related to the probability of an individual with SARS-CoV-2 infection or COVID-19 of the population developing a certain outcome [28]. Interventions, clinical trials, case and qualitative studies were excluded.
We defined multimorbidity as two or more conditions in an individual [13] and included all reports of the association between any two or more conditions and the outcomes of interest. In the literature, multimorbidity is usually assessed by measurements such as disease count, comorbidity or multimorbidity indices or disease combinations (i.e., disease clusters or patterns). These measurements of multimorbidity were considered in the review.
Similarly, we retained studies on frailty which referred to one of the three most commonly used scales, the phenotypic approach by Fried et al. 2001 [29], the frailty index [30] and the clinical frailty scale [31] from the deficit accumulation approach by Rockwood.
In terms of outcomes, the following was considered: short-term outcomes could be SARS-CoV-2 infection confirmed with a test or the information on COVID-19 diagnosis retrieved from hospital records or medico-administrative data, patient hospitalisation, mechanical ventilation, and mortality linked to COVID-19; long-term outcomes could be quality of life, mental health, or functional decline, identified through validated scales.
The systematic search strategy included variations of key terms for multimorbidity, frailty, socioeconomic characteristics, COVID-19 and study design. Supplementary material 1 presents details about the search strategy.
Data sources
The following databases were searched: PubMed, Embase, PsycINFO and World Health Organisation COVID-19 Global literature on coronavirus disease [32].
The initial search was performed on 7 April 2021 to identify literature published from January 2020 onwards. Only peer-reviewed literature published in English was considered. A search update was performed for frailty alone on 1 February 2022 due to the scarcity of evidence identified in the initial search. Supplementary material 2 describes the search strategy used for this update.
Study selection
Screening, data extraction and study quality assessment were performed in pairs. Due to the broader research objectives of the consortium (see Introduction), the record screening phase was performed jointly for biomedical (frailty and multimorbidity) and socioeconomic risk factors. TM and JG acted as the first reviewers and shared the literature during this screening phase. They remained as the first reviewers at the later stage for their respective research topics of biomedical or socioeconomic factors. Eleven colleagues acted as second reviewers (LCB, SMB, LC, RH, RFS, FPB, JC, MA, BV, PB, PJN); five for biomedical part alone. Both teams referred to a third party in the case of disagreements (JCo).
The exclusion criteria for the record screening phase are presented in List 1.
-
List 1: Reasons for exclusion from the systematic review (title/abstract or record screening phase) and number of excluded studies
Criteria for exclusion | Number of excluded studies |
|---|---|
1 = Language other than English | 7 |
2 = Not original research (e.g., editorial, protocol) or no original results | 597 |
3 = Unrelated topic (e.g., studies on individual diseases, outside the review scope) | 8,078 |
4 = Not a population-based study | 534 |
5 = Subpopulation (e.g., pregnant women, health care workers, students) | 74 |
The report screening phase was conducted using the following exclusion criteria (List 2).
-
List 2: Reasons for exclusion from the systematic review (full-text or report screening phase) and number of excluded studies
Reasons for exclusion | Number of excluded studies |
|---|---|
1 = Not a population-based study | 90 |
2 = Unclear SARS-CoV-2 infection diagnosis* | not assigned |
3 = Study does not consider people with frailty or multimorbidity | 88 |
4 = Unclear outcome measurement tool* | not assigned |
5 = Unclear risk factor measurement tool* | not assigned |
6 = Subpopulation (e.g., pregnant women, health care workers, students) | not assigned |
7 = Not original research (e.g., editorial, protocol, review, conference abstract, grey literature), no original results or not peer-reviewed | 28 |
8 = Identical or almost identical population considered in another study with the same outcome | 4 |
9 = Clinical trial or intervention study | not assigned |
10 = Qualitative study | not assigned |
11 = Descriptive study, absence of comparative group and/or no measurement of the association of interest | 16 |
12 = Other (explain)** | 27 |
*even after contacting the study authors.
**full text not found, research question beyond the review scope, ecological study, case series, etc.
The authors were contacted to provide additional details for the studies with missing information. The reference lists of the selected studies were explored (snowballing) to identify additional potential evidence.
Data extraction
For each study, information was extracted into customised tables for each study objective and each outcome and separately for multimorbidity and frailty. The information concerned the study sample characteristics, exposure and outcome details as well as association measures.
Quality and risk of bias assessment
All studies retained for the final synthesis were assessed for quality using the Newcastle–Ottawa scales [33,34,35]. Higher scores indicated better study quality, with a maximum score of 9 points for cohort studies and 10 points for cross-sectional studies.
Results
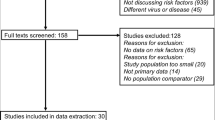
A total of 9,701 records were screened for title and abstract in relation to socioeconomic and biomedical risk factors (Fig. 1), with 411 full-text studies being assessed for inclusion. Of these publications, 267 focused on the biomedical risk factors of multimorbidity and frailty. The search update conducted on frailty on 1 February 2022 retrieved 830 articles: 565 were screened for title and abstract, while only one article was retained. Snowballing for multimorbidity and frailty identified one additional article for frailty.
Finally, 14 studies were retained for data synthesis for multimorbidity (etiological role, n = 2; prognostic role, n = 13) and five for frailty (etiological role, n = 2; prognostic role, n = 4). The outcomes considered for prognosis were SARS-CoV-2 infection, hospitalisation (including ICU admission/ventilation) and mortality, and the maximum follow-up time was 9 months [36].
Details about the reasons for study exclusion are provided in Lists 1 and 2, Fig. 1 and Supplementary material 3.
The average disagreement between the first and second reviewers during the first screening phase for both socioeconomic and biomedical risk factors was 2.1%. The disagreement between two reviewers for the biomedical full-text (report) reading stage was 10%. The third reviewer was consulted 24 times, mainly to provide advice when the representativeness of the study sample was not clear.
Five authors were contacted for additional information, with two of them providing usable information.
The findings are further described below for multimorbidity and frailty separately.
Multimorbidity
Study characteristics
The study criteria were met by 14 studies relating to multimorbidity [36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Two studies described the etiological role of multimorbidity [36, 37], while 13 explored its prognostic role [36, 38,39,40,41,42,43,44,45,46,47,48,49]. The study of Mak et al. [36] complied with both study objectives. Ten studies were cohorts, while four were cross-sectional. The majority of the studies were conducted in high-income countries, with only a few in mid-income settings. Overall, study quality was good; seven studies scored 8 points and one scored 7 points; two cohort studies and three cross-sectional studies scored the maximum of 9 and 10 points, respectively (Table 1 and Supplementary material 4). Five studies were conducted in community settings, four among hospitalised patients, four among outpatients and hospitalised patients and one in the ICU. Only two studies defined multimorbidity, as two or more comorbidities [43] or two or more chronic diseases [38]. The other studies did not specify the definition but instead presented the associations between increasing disease burden (e.g., number of diseases, Charlson Comorbidity Index) and COVID-19 health outcomes. Information on chronic conditions was mainly retrieved from medical records, although the use of a medical coding system was rarely reported. The maximum number of included diseases was 23, while the minimum was 7; only one study did not report the conditions under consideration. COVID-19 diagnosis was usually confirmed by a polymerase chain reaction (PCR) test.
None of the studies reported the long-term health outcomes. Mortality was the most frequently studied outcome. In most studies, adjusted associations with age and sex were the preferred adjustment factors. The largest study included 24,367,476 individuals compared with 308 in the smallest. Table 1 presents the study characteristics.
Etiological role of multimorbidity
The two retained studies [36, 37] were cohorts with large study samples conducted in community settings. Disease burden was measured using the Charlson Comorbidity Index (CCI) (Table 1). Both studies showed a similar direction of the association, namely that the likelihood of poor outcomes increased with a higher CCI score. With each increase in CCI score, the odds ratio (OR) (95% CI) was 1.30 (1.28–1.32) for infection, 1.47 (1.44–1.50) for hospitalisation and 1.53 (1.48–1.59) for mortality [36], and 1.09 (1.06–1.13) and 1.08 (1.03–1.14) for CCI > 0 for hospitalisation and mortality (37), respectively (Table 2).
Prognostic role of multimorbidity
Studies reporting on mortality
Six cohorts and four cross-sectional studies reported on mortality [36, 38, 39, 41, 43, 45,46,47,48,49]. Study samples ranged from 308 to 412,017 participants and covered all age groups. The proportion of females was between 26 and 60%.
Multimorbidity burden was usually based on disease count (six studies), while three studies used CCI and one explored combinations of diseases (dyads or triads composed of diabetes, obesity and hypertension). The number of conditions ranged from 7 to 23, and the data were mainly retrieved from medical records or administrative data (Table 1).
Regardless of the multimorbidity measure, the studies demonstrated an increasing likelihood of mortality with an increasing disease burden. The direction and strength of the association were rather consistent across studies with a significant dose–response relationship. Of the six studies with multimorbidity based on the disease count, two reported multimorbidity as a binary variable (0, 1, ≥ 2), while four studies presented the association for more than two diseases, including one with mortality rate, one with the hazard ratio (HR) and two with OR (Table 2). Odds Ratio for the association between two diseases and mortality in the adjusted models was similar OR (95% CI) of 2.6 (1.7–4.1) [47] and OR (95% CI) 2.51 (2.3–2.7) [43]. Both studies were adjusted for age and sex, while that by Hernández-Vásquez et al. [43] were additionally adjusted for smoking status. The studies also presented unadjusted models with stronger associations; the difference between the unadjusted and adjusted models was particularly high in the study of Reilev et al. [47 with OR (95% CI) of 79.0 (53.5–116.7) for four or more diseases in the unadjusted model versus 5.2 (3.4–8.0) in the adjusted model. Two studies with a continuous CCI score estimated the association with mortality with HR (95% CI) of 1.14 (1.09–1.20) [49] and OR (95% CI) of 1.17 (1.11–1.23) [36]. In the study of Argoty-Pantoja et al. [41], the disease triad of “diabetes & obesity & hypertension” was associated with the highest likelihood of death compared with dyads of these three conditions, with HR (95% CI) of 5.57 (4.54–6.84) and 1.66 (1.54–1.79) among outpatients and hospitalised patients, respectively (Table 2).
Studies reporting on hospitalisation
Only one study reported on hospitalisation [47]. This was a cohort study with 80% community managed cases and 20% hospitalised cases. Disease burden was measured based on disease count (17 conditions). Information on patient health status was retrieved from the administrative and health registries (Table 1). The adjusted risk of hospitalisation increased with the increasing disease count (e.g., for four or more diseases, OR (95% CI) = 3.9 (3.2–4.8)) (Table 2).
Studies reporting on mechanical ventilation
One population-based cohort study reported on mechanical ventilation [49]. The study included 7,327 participants (60% women) with a mean age of 47 ± 19 years. Information on health status was retrieved from administrative data (Table 1). Overall, 1.7% of the total study sample received mechanical ventilation (Table 2). The likelihood of mechanical ventilation increased with increasing CCI score (OR (95% CI) = 1.10 (1.01–1.18)).
Studies reporting on critical or severe illness
One study observed the association between multiple chronic conditions and severe or critical illness (defined based on the WHO guidelines) [40]. A total of 5,685 participants were included in the analysis, with 11% females and a median age (IQR) of 34 (28–43) years. Disease burden was estimated based on disease count (nine chronic conditions) retrieved from electronic medical records. Overall, 1.4% of the population was severely ill, while 0.6% was critically ill (Table 1). Among severely or critically ill patients, 7.1% had two or more diseases (vs 5.7% in the total population). The likelihood of severe or critical illness was higher in the population with more diseases; for example, for three or more diseases versus one disease, OR (95% CI) was 6.16 (3.35–11.32) and 5.43 (3.41–8.63), respectively (Table 2).
Studies reporting on intensive care unit admission or mortality
Two cohort studies reported on ICU admission or mortality [42, 44]. The study of Cardoso et al. [42] had a much larger sample size with 18,647 individuals, a female proportion of 59% and an older population with a median age (IQR) of 50 (36–66) years compared with that of Khan et al. [44] (n = 684, 47% female and median age MD (IQR) = 34 (19)) (Table 1). Both studies found higher OR for ICU admission or mortality with an increased disease count: OR (95% CI) of 2.57 (1.33–4.97) for two or more diseases [44] and 3.57 (2.77–4.60) for two diseases [42] (Table 2).
Frailty
Study characteristics
Five studies reporting on frailty corresponded to our research criteria [36, 37, 39, 50, 51]. Two studies described the etiological role of frailty [36, 37] and four its prognostic role [36, 39, 50]. Mak et al. [36] reported on both objectives. Four studies involved cohorts, two of which scored the maximum of 9 points and two 8 points for quality; the cross-sectional study scored 9 points (Table 1 and Supplementary material 5). One study was conducted in a community setting, three in a hospitalised setting and one in the ICU; the study of Mak et al. [36] was conducted in two settings. All studies indicated the applied definition of frailty. Only one study used an individual frailty assessment based on a questionnaire at inclusion, while the others used a frailty index based on electronic medical records. COVID-19 diagnosis was usually confirmed with a PCR test.
Mortality was the most frequently studied outcome. None of the studies reported the long-term outcomes. The largest study had 24,367,476 individuals compared with 160 in the smallest. Table 1 presents the study characteristics.
Etiological role of frailty
The two retained studies which explored the etiological role of frailty were cohorts, with large study samples involving 410,199 [36] and 24,367,476 individuals [37]. Frailty was assessed using the Hospital Frailty Risk Score computed with ICD-10 codes from hospital records 2 years before the COVID-19 pandemic [36] or via a claims-based frailty index based on ICD-10 codes from hospital records 6 months prior to the study [37] (Table 1). For both studies, the likelihood of poor outcomes increased with a higher probability of frailty: for example, OR (95% CI) associated with an intermediate and high frailty risk were respectively 2.23 (2.03–2.45) and 9.02 (8.10–10.04) for infection, 3.84 (3.41–4.31) and 15.25 (13.45–17.30) for hospitalisation and 5.17 (4.09–6.52) and 20.40 (16.24–25.63) for mortality [36]. OR (95% CI) associated with a 10% increase in the frailty probability was 1.47 (1.45–1.49) for hospitalisation and 1.58 (1.55–1.60) for mortality [37] (Table 3).
Prognostic role of frailty
Of the studies investigating the prognostic role of frailty, three were cohort studies (study samples n = 2,812 [36], n = 91,154 [39] and n = 160 [50]), while one was cross-sectional (study sample n = 18,234 [51]). In Navaratnam et al. [39], 34.8% of the population was aged over 80 years. The population in the study of Mak et al. [36] had a mean age (SD) of 69 (± 8.7) years, which was slightly older than in Hodgson et al. [50] (62 years (IQR: 55–71)), which also had the smallest proportion of females (39.4%). Kundi et al. [51] had the highest proportion of females (53.4%) with a mean population age (SD) of 74 (± 7.4) years (Table 1).
Frailty was assessed using the Hospital Frailty Risk Score [36, 39, 51] or the Clinical Frailty Scale at the time of ICU admission [50] (Table 1).
All studies reported an increased risk of mortality, or mortality or new disability with increasing frailty scores. Three studies that used the Hospital Frailty Risk Score reported similar risks for mortality: OR (95% CI) of 1.53 (1.13–2.05) [36], 1.48 (1.33–1.65) [51] and 1.51 (1.41–1.63) [39] for intermediate or moderate mortality risks. Reported high or severe risks for mortality differed more substantially between the studies: i.e., OR (95% CI) 1.41 (1.04–1.90) [36], 2.08 (1.80–2.41) [51] and 1.80 (1.67–1.94) [39]. All three studies adjusted for sex and age in their models, although Navaratnam et al. [39] adjusted additionally for the deprivation index, ethnicity and date of discharge, and Kundi et al. [51] also for comorbidities, which may be considered to be an overadjustment. In addition, Kundi et al. [51] reported an increased likelihood for ICU admission and invasive mechanical ventilation: for example, OR (95% CI) of 1.38 (1.24–1.53) and 1.77 (1.53–2.05) for invasive mechanical ventilation with intermediate- and high-risk scores, respectively (Table 2).
Discussion
Summary and discussion of the findings
This systematic review adopted a population-based approach and investigated the etiological and prognostic roles of multimorbidity and frailty in terms of COVID-19 health outcomes in the early years of the pandemic. Literature was scarce, especially for frailty, and the studies focused on short-term outcomes, mostly reporting on mortality. An increased risk of poorer outcomes was associated with higher multimorbidity or frailty levels, which was observed across all measurements of multimorbidity and frailty. We did not identify any studies examining the long-term outcomes.
To account for multiple conditions, researchers most commonly used disease count and Charlson comorbidity index. Only one study examined the association between disease combinations and COVID-19 outcomes. However, it was limited to three cardiometabolic conditions and thus cannot be considered representative of the population with multimorbidity, although it was retained based on the cut-off point of two or more conditions for multimorbidity to emphasise the pertinence of exploring the effects of disease combinations. Identifying the most frequent and most harmful combinations is essential, as the joint effect of disease patterns may be stronger than their individual additive effects [52]. Despite the broad body of evidence on the association between individual chronic conditions and COVID-19-related outcomes [18], studies on patients with multiple conditions are clearly less numerous, particularly among population-representative samples. Among the 14 studies included in this review, only two focused on multimorbidity by specifying the multimorbidity definitions they used.
Similarly, all studies on frailty in our review reported a significant association between higher frailty scores and poorer outcomes. While population-representative studies are scarce, there is substantial evidence with smaller hospital-based samples [53, 54]. These studies confirm the strong association between frailty levels and poorer short-term outcomes such as mortality, although they may underestimate the strength of the association, as they are based on severe cases. The study of Mak et al. [36], which provided estimates at the population level and among hospitalised patients, clearly demonstrated a stronger association between frailty and mortality at the population level. The main constraint of the evidence provided by this review regarding frailty relates to the fact that most included studies used electronic frailty scores. These scores are not based on face-to-face examination nor on assessment of individuals' functional and cognitive performances. This information would improve the accuracy and sensitivity of frailty measurement and provide more robust estimations of associations with COVID-19 outcomes. In addition, the identification of frailty at the primary care level could improve prevention in this population during this or similar public health emergencies in the future. There is still a lack of well-performed population-based studies that assess the actual association between frailty in the community-based population and COVID-19 severity.
Our results present the state of the evidence in the early years of the COVID-19 pandemic. Since our review, several studies on population representative samples have been released for multimorbidity [55,56,57,58,59,60,61,62] and frailty [63,64,65]. They also consider earlier waves of the pandemic (study period between January 2020 and July 2021) and address short-term outcomes, notably in terms of infection, hospitalisation, ICU admission and mortality. The studies support our findings underlying the higher risk of poorer outcomes with the higher number of diseases or with a CCI score increment [55, 56, 58, 59, 61, 62], with increasing frailty [63,64,65] and for certain disease patterns such as cardiometabolic or cardiovascular patterns, which presented a stronger association with infection or infection severity [57, 60]. Regarding the risk of infection, however, the findings seem to be less conclusive, for e.g., Catalano et al. [58] showed the lesser likelihood of infection for patients with multimorbidity, even though they were tested more often, which may potentially be explained by their better compliance with the restrictive measures; nevertheless, in the same study, the risk of hospitalisation, ICU admission or death was higher for patients with multimorbidity. The number of emerging studies on population representative samples seems encouraging, although it is clear that making this evidence available requires time.
Study limitations
Only quantitative studies were considered. As case and qualitative study designs require different approaches for evidence synthesis [66,67,68], they were excluded for feasibility reasons. Further, we only included scientific publications in English, which may have led to the omission of population-based studies published in other languages. Despite a pilot test being conducted prior to the study and regular weekly meetings to reduce heterogeneity during the screening process [23], disagreements between two reviewers were frequent during the full-text reading phase. However, the quality check was ensured through regular consultations with the third reviewer. The instruments used to assess multimorbidity and frailty varied across the studies, thus making comparisons difficult. This along with other variations such as the insufficient number of studies using the same outcome and the association metrics precluded the possibility of performing meta-analysis, which required a minimum of four comparable studies as indicated in the protocol of the study published earlier [23]. The number and type of diseases also differed across studies, although the most prevalent chronic conditions were included. The use of a medical coding system such as the International Classification of Diseases (ICD) was often not reported. In addition, the study settings differed across the studies (e.g., community setting, hospital setting, ICU), although the population representativeness of the sample was always required with the inclusion of all community or hospitalised cases, for instance. In this regard, studies undertaken in the same setting may be more comparable. The population representativeness of the sample was difficult to determine on several occasions during the screening process, which may have led to the omission of some evidence. The studies most frequently controlled for age and sex, although factors such as poverty level, ethnicity, influenza vaccination status or others were occasionally considered. Where unadjusted models were also presented, the adjusted models showed a less strong association between the risk factors and outcomes, thus indicating that sociodemographic, socioeconomic, biological or behavioural factors may also influence these associations. Certain studies adjusted for individual diseases or frailty in addition to multimorbidity, thus leading to overadjustments and minimising the strengths of the association in the resulting models.
Recommendations for research and policy
Repeated exposure, different virus variants and vaccination in the later years of the pandemic may have changed the landscape of infection. The intention of our work was to underline the relevance of estimations at the population level to guide public health decisions; here we provide the existence of those estimations for discussed health groups during the initial phases of the pandemic. The studies which discuss the later waves of SARS-CoV-2 infection will be relevant in observing the potential evolution of the associations presented here and in comparing their direction and strength with those of the later waves, while taking into consideration more recent virus variants and the effects of vaccination. Considering that the circulation of variants and vaccination programs differed across geographical regions, recent population-representative studies performed in the same regions and the same settings as those discussed here would be particularly valuable to compare the findings. Researchers should be encouraged to adjust for age and sex at a minimum. As poor socioeconomic characteristics also influence multimorbidity and frailty [52, 69,70,71] as well as COVID-19 outcomes [72], they should also be considered in models to provide more precise estimates. The majority of the studies identified in our review were conducted in high-income countries, with only a few being performed in middle-income settings. Bearing in mind that life expectancy is increasing worldwide and even at a faster rate in less-off parts of the world [73], given the challenges posed by ageing societies, low-income countries, which endured the most devastating effects of the COVID-19 pandemic, should be urged to estimate the relationships investigated here. Multimorbidity manifests differently across age and sex groups [74], as appears to be the case for the COVID-19 outcomes [11, 75]. The studies identified in our review did not provide stratified estimates for these groups. Observing the associations between multimorbidity or frailty and COVID-19 outcomes separately for men and women and for different age groups would provide more detailed information about the effects of the pandemic.
Unfortunately, no studies on the long-term impacts were identified. Considering the uncertainty of the COVID-19 pandemic during the early years, the concerns about short-term outcomes seems reasonable. It is nevertheless increasingly obvious that the COVID-19 pandemic has left long-lasting consequences on mental health [8], quality of life [76], daily functioning and work capacities [77]. As these consequences risk overburdening the national health and economic systems in the future, they should be recognized and managed in a timely manner.
Lastly, biological mechanisms such as the weakened physiological capacities of multiple organ systems in patients with frailty and/or multimorbidity certainly play an important role in the association with COVID-19 outcomes. Altered biological functioning may increase susceptibility to infection and the likelihood of poorer outcomes. These mechanisms and their potential interaction with COVID-19 pathophysiology merit more research.
Conclusion
This review provides clear, coherent but limited evidence on the association between increased disease and frailty burden and poorer COVID-19 outcomes in population-representative samples for the early years of the pandemic. Future studies should use the same tools for exposure and outcomes to the feasible extent, to ensure better comparability and certainty in the strength of the associations.
In ageing societies, multimorbidity and frailty represent growing challenges in both developed and developing contexts. Single disease-oriented healthcare systems are already struggling to meet the health and financial demands caused by these complex conditions, and overlooking their importance in the current context risks compounding these issues with the consequences of infectious diseases. The magnitude of these repercussions should be thoroughly explored to guide adequate public health decisions and reduce the impact of COVID-19. Any lessons drawn now may help in the management of any future health crisis should it emerge.
Data availability
This systematic review used only already published data.
References
Mehraeen E, Oliaei S, SeyedAlinaghi S et al (2022) COVID-19 in Pediatrics: a systematic review of current knowledge and practice. Infect Disord Drug Targets 22:e290921196908
Cui X, Zhao Z, Zhang T et al (2021) A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol 93:1057–1069
Alves VP, Casemiro FG, Araujo BG et al (2021) Factors associated with mortality among elderly people in the COVID-19 pandemic (SARS-CoV-2): a systematic review and meta-analysis. Int J Environ Res Public Health. 18:8008
SeyedAlinaghi S, Karimi A, Barzegary A et al (2022) COVID-19 mortality in patients with immunodeficiency and its predictors: a systematic review. Eur J Med Res 27:195
Kremer D, Pieters TT, Verhaar MC et al (2021) A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: Lessons to be learned. Am J Transplant 21:3936–3945
Gómez-Ochoa SA, Franco OH, Rojas LZ et al (2021) COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 190:161–175
Mirbeyk M, Saghazadeh A, Rezaei N (2021) A systematic review of pregnant women with COVID-19 and their neonates. Arch Gynecol Obstet 304:5–38
SeyedAlinaghi S, Karimi A, Shobeiri P et al (2021) Psychological symptoms of COVID-19 epidemic: a systematic review of current evidence. Psihologija 54:173–192
Vindegaard N, Benros ME (2020) COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun 89:531–542
Welch C (2021) Age and frailty are independently associated with increased COVID-19 mortality and increased care needs in survivors: results of an international multi-centre study. Age Ageing 50:617–630
Himmels JPW, Borge TC, Brurberg KG, Gravningen KM (2021) COVID-19: COVID-19 and risk factors for hospital admission, severe disease and death [Covid-19 og risikofaktorer for sykehusinnleggelse, alvorlig sykdom og død - en hurtigoversikt, fjerde oppdatering. Hurtigoversikt 2021] Oslo: Norwegian Institute of Public Health
Chen Y, Klein SL, Garibaldi BT et al (2021) Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev 65:101205
van den Akker M, Buntinx F, Knottnerus JA (1996) Comorbidity or multimorbidity: what’s in a name? A review of literature. European Journal of General Practice 2:65–70
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancet 381:752–762
Marengoni A, Angleman S, Melis R et al (2011) Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 10:430–439
Veronese N, Custodero C, Cella A et al (2021) Prevalence of multidimensional frailty and pre-frailty in older people in different settings: a systematic review and meta-analysis. Ageing Res Rev 72:101498
Eurostat EU. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments#Older_people_.E2.80.94_population_overview
Tisminetzky M, Delude C, Hebert T et al (2022) Age, multiple chronic conditions, and COVID-19: a literature review. J Gerontol A Biol Sci Med Sci 77:872–878
Whitty CJM, MacEwen C, Goddard A et al (2020) Rising to the challenge of multimorbidity. BMJ 368:l6964
Yang Y, Luo K, Jiang Y et al (2021) The impact of frailty on COVID-19 outcomes: a systematic review and meta-analysis of 16 cohort studies. J Nutr Health Aging 25:702–709
Guan WJ, Liang WH, Zhao Y et al (2020) Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55:2001227
Mair FS, Foster HM, Nicholl BI (2020) Multimorbidity and the COVID-19 pandemic - An urgent call to action. J Comorb 10:2235042x20961676
Makovski TT, Ghattas J, Monnier Besnard S et al (2022) Aetiological and prognostic roles of frailty, multimorbidity and socioeconomic characteristics in the development of SARS-CoV-2 health outcomes: protocol for systematic reviews of population-based studies. BMJ Open 12:e063573
European Commission: Horizon (2020) Population Health Information Research Infrastructure (PHIRI), 2020. https://cordis.europa.eu/project/id/101018317. Accessed 1 Feb 2024
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647
Gail MH (1998) Population based study. In: Armitage P, Colton T (eds) Encyclopedia of biostatistics. Wiley, Chichester, p 3450
Lieb R (2013) Population-based study. In: Gellman MD, Turner JR (eds) Encyclopedia of behavioral medicine. Springer New York, NY, New York, pp 1507–1508
Kleinbaum DG, Kupper LL, Morgenstern H (1982) Epidemiologic research: principles and quantitative methods. Lifetime Learning Publications, Belmont, California
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. Sci World J 1:323–336
Rockwood K, Song X, MacKnight C et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173:489–495
World Health Organisation (WHO) COVID-19 Global literature on coronavirus disease, 2022. https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/. Accessed 1 Feb 2022
Wells GA, Shea B, O'Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Feb 2024
Herzog R, Álvarez-Pasquin MJ, Díaz C et al (2013) Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health 13:154
Modesti PA, Reboldi G, Cappuccio FP et al (2016) Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PLoS ONE 11:e0147601
Mak JKL, Kuja-Halkola R, Wang Y et al (2021) Frailty and comorbidity in predicting community COVID-19 mortality in the U.K. Biobank: The effect of sampling. J Am Geriatr Soc 45:1270
Izurieta HS, Graham DJ, Jiao Y et al (2020) Natural history of COVID-19: risk factors for hospitalizations and deaths among >26 million U.S. Medicare beneficiaries. J Infec Dis 223:945–956
Ticinesi A, Nouvenne A, Cerundolo N et al (2021) Trends of COVID-19 admissions in an Italian hub during the pandemic peak: large retrospective study focused on older subjects. J Clin Med 10:1115
Navaratnam AV, Gray WK, Day J et al (2021) Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med 9:397–406
Al Kuwari HM, Abdul Rahim HF, Abu-Raddad LJ et al (2020) Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February-18 April 2020. BMJ Open 10:e040428
Argoty-Pantoja A, Robles-Rivera K, Rivera-Paredez B et al (2021) COVID-19 fatality in Mexico’s indigenous populations. Public Health 193:69–75
Cardoso FS, Papoila AL, Machado RS et al (2020) Age, sex, and comorbidities predict ICU admission or mortality in cases with SARS-CoV2 infection: a population-based cohort study. Crit Care 24:465
Hernández-Vásquez A, Azañedo D, Vargas-Fernández R et al (2020) Association of comorbidities with pneumonia and death among COVID-19 patients in Mexico: a nationwide cross-sectional study. J Prev Med Public Health 53:211–219
Khan A, Althunayyan S, Alsofayan Y et al (2020) Risk factors associated with worse outcomes in COVID-19: a retrospective study in Saudi Arabia. East Mediterr Health J 26:1371–1380
Haase N, Plovsing R, Christensen S et al (2020) Characteristics, interventions, and longer term outcomes of COVID-19 ICU patients in Denmark-A nationwide, observational study. Acta Anaesthesiol Scand 65:68–75
Millán-Guerrero RO, Caballero-Hoyos R, Monárrez-Espino J (2020) Poverty and survival from COVID-19 in Mexico. J Public Health (Oxf) 43:437–444
Reilev M, Kristensen KB, Pottegård A et al (2020) Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol 49:1468–1481
Sousa BLA, Sampaio-Carneiro M, de Carvalho WB et al (2020) Differences among severe cases of Sars-CoV-2, influenza, and other respiratory viral infections in pediatric patients: symptoms outcomes and preexisting comorbidities. Clinics 75:e2273-e
McQueenie R, Foster HME, Jani BD et al (2020) Multimorbidity, polypharmacy, and COVID-19 infection within the UK Biobank cohort. PLoS ONE 15:e0238091
Hodgson CL, Higgins AM, Bailey MJ et al (2021) The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care 25:1–12
Kundi H, Çetin E, Canpolat U et al (2020) The role of frailty on adverse outcomes among older Patients with COVID-19. J Infect 81:944–951
Coste J, Valderas JM, Carcaillon-Bentata L (2021) Estimating and characterizing the burden of multimorbidity in the community: A comprehensive multistep analysis of two large nationwide representative surveys in France. PLoS Med 18:e1003584
Cosco TD, Best J, Davis D et al (2021) What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing 50:608–616
Pranata R, Henrina J, Lim MA et al (2021) Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch Gerontol Geriatr 93:104324
Agrawal U, Azcoaga-Lorenzo A, Fagbamigbe AF et al (2022) Association between multimorbidity and mortality in a cohort of patients admitted to hospital with COVID-19 in Scotland. J R Soc Med 115:22–30
Cardoso JP, Calazans MIP, Carneiro A et al (2022) Association between multimorbidity, intensive care unit admission, and death in patients with COVID-19 in Brazil: a cross-section study, 2020. Sao Paulo Med J 141:e2022226
Carmona-Pírez J, Gimeno-Miguel A, Bliek-Bueno K et al (2022) Identifying multimorbidity profiles associated with COVID-19 severity in chronic patients using network analysis in the PRECOVID Study. Sci Rep 12:2831
Catalano A, Dansero L, Gilcrease W et al (2023) Multimorbidity and SARS-CoV-2-Related Outcomes: analysis of a cohort of Italian patients. JMIR Public Health Surveill 9:e41404
Guillon A, Laurent E, Duclos A et al (2021) Case fatality inequalities of critically ill COVID-19 patients according to patient-, hospital- and region-related factors: a French nationwide study. Ann Intensive Care 11:127
Huang YT, Steptoe A, Patel RS et al (2023) the impact of long-term conditions and comorbidity patterns on COVID-19 Infection and Hospitalisation: a cohort study. Gerontology 69:1200–1210
Kone AP, Martin L, Scharf D et al (2023) The impact of multimorbidity on severe COVID-19 outcomes in community and congregate settings. Dialogues Health 2:100128
Prado PRD, Gimenes FRE, Lima MVM et al (2021) Risk factors for death due to COVID-19, in the state of Acre, Brazil, 2020: a retrospective cohort study. Epidemiol Serv Saude 30:e2020676
Andrew MK, Godin J, LeBlanc J et al (2022) Older age and frailty are associated with higher mortality but lower ICU admission with COVID-19. Can Geriatr J 25:183–196
Wijeysundera HC, Abdel-Qadir H, Qiu F et al (2022) Relationship of frailty with excess mortality during the COVID-19 pandemic: a population-level study in Ontario. Canada Aging Clin Exp Res 34:2557–2565
Zhu Y, Sealy MJ, Jager-Wittenaar H et al (2022) Frailty and risk of hospitalization from COVID-19 infection among older adults: evidence from the Dutch Lifelines COVID-19 Cohort study. Aging Clin Exp Res 34:2693–2702
Lewin S, Glenton C, Munthe-Kaas H et al (2015) Using qualitative evidence in decision making for health and social interventions: an approach to assess confidence in findings from qualitative evidence syntheses (GRADE-CERQual). PLoS Med 12:e1001895
Tong A, Flemming K, McInnes E et al (2012) Enhancing transparency in reporting the synthesis of qualitative research: ENTREQ. BMC Med Res Methodol 12:1–8
World Health Organisation Regional Office for Europe Guide to qualitative evidence synthesis: evidence-informed policy-making using research in the EVIPNET framework, 2021; Document number: WHO/EURO:2021-2272-42027-57819. Accessed 1 Feb 2024
Pathirana TI, Jackson CA (2018) Socioeconomic status and multimorbidity: a systematic review and meta-analysis. Aust N Z J Public Health 42:186–194
Franse CB, van Grieken A, Qin L et al (2017) Socioeconomic inequalities in frailty and frailty components among community-dwelling older citizens. PLoS ONE 12:e0187946
Barnett K, Mercer SW, Norbury M et al (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380:37–43
Magesh S, John D, Li WT et al (2021) Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta-analysis. JAMA Netw Open 4:e2134147-e
World Health Organisation (WHO), Ageing and Health, 2022. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed 1 Feb 2024
MacRae C, Mercer SW, Henderson D et al (2023) Age, sex, and socioeconomic differences in multimorbidity measured in four ways: UK primary care cross-sectional analysis. Br J Gen Pract 73:e249–e256
Sieurin J, Brandén G, Magnusson C et al (2022) A population-based cohort study of sex and risk of severe outcomes in covid-19. Eur J Epidemiol 37:1159–1169
Poudel AN, Zhu S, Cooper N et al (2021) Impact of Covid-19 on health-related quality of life of patients: a structured review. PLoS ONE 16:e0259164
Godeau D, Petit A, Richard I et al (2021) Return-to-work, disabilities and occupational health in the age of COVID-19. Scand J Work Environ Health 47:408–409
Acknowledgements
We thank Dr. Laetitia Haroutunian, a documentation expert from Santé publique France for her invaluable guidance in formulating the search strategy.
Funding
The study was performed as part of the Population Health Information Research Infrastructure (PHIRI) project funded by the European Union’s (EU) Horizon 2020 research and innovation program under the grant agreement no. 101018317 (available from: https://cordis.europa.eu/project/id/101018317). Partners from five EU countries worked jointly on this project. The funding body was not involved in the study design, in the collection, analysis and interpretation of data or in the writing of the manuscript. The content of this publication represents the views of the authors alone and not those of the European Commission or any other body of the European Union. The European Commission does not take any responsibility for the use of this document and any information that it contains.
Author information
Authors and Affiliations
Contributions
LCB, JCo, BD, PJN and MJF identified the research questions. All authors were involved in the conceptualisation of the search strategy together with documentation expert LH who advised on the databases, keywords selection and search optimisation. TM and JG acted as first reviewers, while LCB, SMB, LC, RH, RFS, FPB, JC, MA, BV, PB, PJN shared the role of the second reviewer. JCo acted as a third party and resolved all disagreements. TM, JG, JCo, BD and LCB formulated the first version of the manuscript. All authors revised and approved its final version.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing interests.
Human and animal rights
Not applicable, as this systematic review used already published data.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 Supplementary material 2: Search strategy for the update on frailty (PDF 415 KB)
40520_2023_2685_MOESM3_ESM.xlsx
Supplementary file3 Supplementary material 3: Excluded studies and reasons for exclusion (full-text or report screening phase) (XLSX 47 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makovski, T.T., Ghattas, J., Monnier-Besnard, S. et al. Multimorbidity and frailty are associated with poorer SARS-CoV-2-related outcomes: systematic review of population-based studies. Aging Clin Exp Res 36, 40 (2024). https://doi.org/10.1007/s40520-023-02685-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-023-02685-4