Abstract
Objective
The prevalence of obesity by fat percentage has seen a steady increase in older adults in recent years, secondary to increases in fat mass in body composition, even in healthy aging. Malnutrition is a common geriatric syndrome with serious clinical outcomes. Increases in fat mass and waist circumference with healthy aging should not prevent the risk of malnutrition from being masked. Malnutrition is often ignored in obese older people due to low BMI cut-off values in many screening tests. The present study seeks to raise awareness of the need to assess the frequency of undernutrition and related factors in obese older adults.
Methods
The data of 2013 community-dwelling patients aged ≥ 60 years who applied to a university geriatrics outpatient clinic between April 2012 and November 2022 were analyzed retrospectively, of which 296 were found to be obese based on fat percentage and were included in the study. Demographic data and the presence of any geriatric syndromes were obtained retrospectively from the patient files, functional status was assessed using the KATZ Activities of Daily Living (ADL) Scale and the LAWTON-BRODY Instrumental Activities of Daily Living Scale (IADL); frailty was screened using FRAIL-scale; and the sample was assessed for malnutrition using the Mini Nutritional Assessment-Short Form (MNA-SF), with undernutrition defined as an MNA-SF score of \(\le 11.\) The patients’ fat percentage and weight were measured using a bioimpedance analyzer. Fatty obesity was defined using the Zoico methodology (fat percentage \(\ge\) 27.3% for males, \(\ge\) 40.7% for females)\(,\) handgrip strength (HGS) was measured using a hand dynamometer, and probable sarcopenia was defined as low HGS based on regional cut-off values (35 kg for males, 20 kg for females).
Results
The mean age of the 296 fatty obese older adults (102 males/194 females) was 74.4 + 6.5 years, and the median fat was 42.2% (27.4–59.5). Undernutrition was detected in 19.6% of the patients based on MNA-SF screening. A univariate analysis revealed age, sex, educational status, daily physical activity status, depression, difficulty in swallowing, chewing difficulty, probable sarcopenia, number of chronic diseases, and IADL to be associated with undernutrition, while a multivariate logistic regression analysis revealed depression [OR = 3.662, 95% CI (1.448–9.013), p = 0.005] and daily physical activity status [OR:0.601, 95% CI (0.417–0.867), p = 0.006] to be independently associated with malnutrition in obese older adults based on fat percentage.
Conclusion
The present study clarifies the significance of undernutrition in obese older adults also in our country, and recommends undernutrition screening to be carried out, by fat percentage, on obese older adults, especially with depression and low daily physical activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the prevalence of obesity has seen a marked increase in all age groups, including older adults [1,2,3]. In addition to the associated risks of cancer and cardiovascular disease, as common negative consequences of obesity, studies in literature have also reported obesity to be associated with impaired functionality and cognition in older adults [4,5,6,7]. Various changes occur in the body’s metabolism and composition with aging, the risk components of which increase the likelihood of obesity. Compensatory changes occur in body composition secondary to decreases in the metabolism rate and mobility, and these physiological changes, along with the aging process, lead to an increase in fat in the body composition and a decrease in muscle mass [8]. The most commonly used definition of obesity is the one put forward by the World Health Organization (WHO), which defines obesity as Body Mass Index (BMI) \(\ge\) 30 kg/m2 [9]. The BMI definition cannot define the presence or absence of sarcopenia in the older adults defined as obese because it does not include the body’s muscle and fat mass. Sarcopenia is an important geriatric syndrome that is the result of malnutrition. The BMI definition ignores undernutrition and sarcopenia in older adults defined as obese for these reasons. However, the definition made according to BMI has revealed the necessity of new definitions for sarcopenic older adults, whose body fat percentage increases and muscle mass decreases with aging.
This has resulted in the inclusion in literature of obesity definitions based on body fat percentage [10, 11], including Zoico et al., who suggest that a fat percentile above the 60th percentile should be defined as obesity [10, 11]. The body fat percentage of older adults may vary due to regional differences. In a study of obesity in older adults in our region in recent years, obesity was defined as a body fat percentage of \(\ge\) 40.7% in women and \(\ge\) 27.3% in men based on the Zoico methodology, drawing attention to the need to define obesity in older adults with consideration of population-specific body fat percentages [11].
Malnutrition can have serious adverse clinical consequences for older adults [12,13,14,15,16], including frailty, sarcopenia, falls, hospitalization, loss of functionality and dependency [12,13,14,15,16,17,18].
Malnutrition is a significant geriatric syndrome due to its adverse outcomes and its bidirectional association with sarcopenia [17,18,19], and evaluating obesity based solely on BMI while neglecting the malnutrition risk in sarcopenic obese geriatric patients can have considerable consequences.
The present study investigates the prevalence of undernutrition and the associated factors in older adults with fatty obesity, defined based on the cut-off values specific to our region.
Materials and method
Study design and population
For this retrospective study, the data of 2013 community-dwelling patients aged ≥ 60 years who applied to a university geriatrics outpatient clinic between April 2012 and November 2022 were assessed, however, 1717 were subsequently excluded due to a lack of consent, a lack of trained personnel, insufficient evaluations at outpatient presentation and inability to undergo procedures due to health problems, including sequelae neurological deficit, neuropathy, pacemaker, severe osteoarthritis, severe hearing problems, advanced dementia and general conditional disorders. Patients aged ≥ 60 years who gave consent for their inclusion in the study and who were able to perform the measurement procedures were included in the study. The study population comprised 296 participants (Fig. 1), whose demographic data, including age, sex, geriatric syndromes, chronic diseases, and measurements, were obtained retrospectively from the patient files. Prior approval for the study was granted by the Local University Faculty of Medicine Clinical Research Ethics Committee (Reference number: 2023/222).
Assessment
The participants’ age, sex, education status, marital status, smoking status, the number of diseases/drugs, and geriatric syndromes (difficulty in swallowing, chewing difficulty, falls, urinary incontinence, fecal incontinence, constipation, sleep disorders, chronic pain, frailty, probable sarcopenia, functional status and quality of life) were obtained from the patient files, as well as any chronic diseases.
Polypharmacy was defined as using ≥ 5 drugs [20] and the participants’ daily physical activity status was measured based on a multiple-choice questionnaire with the options: never, sometimes, 1–2 times per week and every day. The participants were questioned for any swallowing difficulties, chewing difficulties, falls, urinary incontinence, fecal incontinence, constipation, sleep problems and chronic pain. Chronic pain was assessed by asking the participants whether they had experienced pain for more than six months, and they were asked to provide a pain intensity score on a scale of 0–10 using the Visual Analog Scale (VAS) (0: least severe pain, 10: most severe pain in their life) [21]. Functional status was evaluated using the six-item KATZ Activities of Daily Living (ADL) Scale and the eight-item LAWTON-BRODY Instrumental Activities of Daily Living Scale (IADL) [22,23,24]. The participants scored 0 points for activities that could not be performed or that could be performed only with assistance, and 1 point for activities that could be performed alone. The ADL scale produces a total score in the range of 0 (dependent) to 6 (independent), while the IADL scale produces a total score in the range of 0 (dependent) to 8 (independent) [22,23,24]. Frailty was screened using the five-item FRAIL-scale, measuring fatigue, resistance, ambulation, illnesses and weight loss, in which a score of 0 indicated robust, scores of 1–2 indicated pre-frail and 3–5 indicated frail [25]. Quality of life was assessed using the European Quality-5 Dimension visual analog scale (EQ-5 VAS) [26], which is scored using a visual scale in which 0 denotes the worst health condition, and 100 the best health condition [26].
Measurements
The height, calf circumference, mid-upper arm circumference, hip circumference, and waist circumference of the participants were measured using a regular stadiometer, and Body Mass Index (BMI) was calculated using the formula: body weight (kg)/height2 (m2) [9]. Handgrip strength (HGS) was measured using a Jamar hydraulic hand dynamometer. While seated in a standard backed chair, the respondent was asked to squeeze the dynamometer as hard as they could for 3 s in each hand with their arm next to their body, the elbow flexed 90°, and with the forearm and wrist in a neutral position, and the highest measured value was recorded [27]. Body composition measurements were made through a bioimpedance analysis (BIA, Tanita-BC532). After fat free mass measurements were achieved from the BIA device, skeletal muscle mass was computed with the equation below.
[Skeletal muscle mass = (Fat-free mass*0.566)] [10, 11, 28].
Undernutrition definition
Malnutrition was screened using the Mini Nutritional Assessment-Short Form (MNA-SF), and undernutrition was defined as an MNA-SF score \(\le 11 \left[29\right].\)
Obesity definition
Fat percentage and weight were measured using a BIA, and fatty obesity was defined based on the Zoico methodology (fat percentage \(\ge\) 27.3% in males, 40.7% in females) [10, 11]\(.\)
Probable sarcopenia definition
Probable sarcopenia was defined as low HGS based on regional cut-off values (35 kg for males, 20 kg for females) [30, 31]\(.\)
Statistical analysis
The normality of continuous data was analyzed with a Kolmogorov–Smirnov test. For the descriptive statistics, continuous variables were expressed as mean ± standard deviation, median, and minimum–maximum values, while categorical variables were expressed as numbers (of subjects) and percentages. The differences between groups were determined with an independent samples t test, a Mann–Whitney U test and a Wilcoxon test. The Chi-square test and Fisher’s exact test for 2 × 2 probability tables suitable for categorical variables. A multivariate logistic regression analysis using the Enter method was used to determine the independent factors associated with undernutrition among the factors identified as significant in the univariate analyses. Multicollinearity was checked among the selected parameters, and Odds ratio (OR) and 95% confidence interval (CI) were used to express the associations. The fitness of the model was evaluated with a Hosmer–Lemeshow goodness-fit test. p values were based on two-sided tests and were accepted as statistically significant if < 0.05. The statistical evaluation of the study data was carried out using IBM SPSS Statistics (Version 20.0. Armonk, NY: IBM Corp.)
Results
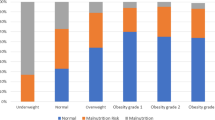
Included in the study were 296 fatty obese older adults (102 male/194 female) [mean age:74.4 + 6.5 years (61–92)], among which 19.6% were undernourished (Fig. 1). The median BMI of the study group was 32.8 (21.7–58.7) kg/m2 (Table 1).
Univariate analyses revealed a statistically significant association between undernutrition and age (p = 0.037), sex (p = 0.031), education status (p = 0.027), daily physical activity (p < 0.001), depression (p = 0.001), chronic kidney disease (p = 0.021), difficulty in swallowing (p = 0.009), chewing difficulty (p = 0.002), urinary incontinence (p = 0.006), constipation (p = 0.004), frailty (p < 0.001), probable sarcopenia (35/20 kg) (p < 0.019), number of diseases (p = 0.026), number of drugs (p = 0.001), ADL (p < 0.001), IADL (p < 0.001) (Table 1).
Univariate analyses revealed a statistically significant association between undernutrition and hand grip strength (p = 0.001), fat free mass (p < 0.001), skeletal muscle mass (p < 0.001), calf circumference (p = 0.034) and mid-upper arm circumference (p = 0.038). However, no statistically significant relationship was found between BMI (p = 0.282) and undernutrition (Table 2).
Undernutrition was found to be statistically related with fat free mass (p < 0.001), skeletal muscle mass (p < 0.001). Also, statistically significant relationship was found between fat free mass (p < 0.001) and skeletal muscle mass (p < 0.001) with the hand grip strength.
In multivariate analyses including factors independently associated with undernutrition in fatty obese older adults were adjusted for age, gender, education status, daily physical activity status, chronic kidney disease, depression, difficulty in swallowing, chewing difficulty, probable sarcopenia (35/20 kg), number of chronic diseases, IADL. After adjustment independently associated factors found with undernutrition the presence of depression (OR 3.662 p = 0.005), and daily physical activity status (OR 0.601, p = 0.006) in multivariate analyses (Table 3).
Discussion
The results of the present study of 296 older adults identified as obese based on their fat percentage revealed a risk of malnutrition in 19.6%, and univariate analyses identified many factors (age, sex, education status, daily physical activity, chronic kidney disease, depression, difficulty in swallowing, chewing difficulty, urinary incontinence, constipation, frailty, probable sarcopenia (35/20 kg), number of diseases, number of drugs, ADL, IADL, calf circumference, mid-upper arm circumference) that were independently associated with malnutrition risk.
In the multivariate analyses, presence of depression and daily physical activity status were identified as being associated with malnutrition risk in older obese adults. The results of the present study identified malnutrition in 19.6% of the obese older adult respondents based on fat percentage. The most common definition of obesity in older adults and in all societies is based on BMI, as recommended by a WHO Expert Committee [9], and most studies of older adults are based on this definition. A review of literature reveals malnutrition risk in obese older adults is most commonly based on the BMI definition, and that the prevalence of malnutrition varies from one society to another [32,33,34,35,36]. According to the findings of one study, even in Central Africa, where the risk of malnutrition is high, the prevalence of obesity among older adults is 8.8% [32]. In Turconi et al.’s study of 184 older adults over the age of 60 years in Italy, 39.1% were found to be obese based on the BMI definition, although most of the participants had a low socioeconomic level [33], and 12% of the older participants in the study were found to be malnourished according to MNA-Long Form [33]. In Kaiser et al.’s validation study for the Mini Nutritional Test Short Form (MNA-SF), the risk of malnutrition was ignored in 6% of the 656 participants when the risk of malnutrition was evaluated based on BMI [34].
In Bahat et al.’s study of nursing home patients, the prevalence of nursing home residents with a BMI ≥ 25 was found to be 56.6%, and of these overweight + obese adults, 17.3% were found to be at risk of undernutrition. In addition, the prevalence of obesity among older adults living in nursing homes was 21.6%, and 9.1% were found to be at risk of undernutrition [35]. Soysal et al.’s cross-sectional retrospective studies of 1911 older adults revealed a prevalence of obesity of 48.7%, and 32.3% who were at risk of undernutrition [36].
In their study, Özkaya et al. found that 187 of the 596 older adult participants in their study under 1-year follow-up in the outpatient clinic were overweight + obese, and 49.7% were at undernutrition risk [37]. Our results differ significantly from those of other studies, which may be attributed to differences in the definition of obesity. The definition of obesity recommended by WHO, which is in common use around the world, ignores the changing body composition of older adults, and so obesity in the present study was defined using the Zoico methodology, which takes into account the changes in body composition with aging, and has been adopted in other studies to date [10, 11, 38, 39].
In the first of these studies, 167 community-dwelling women aged 67–78 were compared with 120 premenopausal Italian women between the ages of 20–50, and those with a fat percentile above the 60th percentile were identified as obese [10].
In another study conducted in France involving 1,308 healthy women aged 75 and over, those with a fat percentile over the 60th percentile were defined as obese. When they defined obesity, this as having a body fat percentage above 40%, around 36% of the participants were found to be obese based on their body fat percentage [38]. A study by Kim et al. in Korea involving 526 participants, obesity was defined as above the 60th percentile of fat percentage measured by Dual-Energy X-Ray Absorptiometry (DXA), and defined as body fat percentages above 20.21% and 31.71% for males and females, respectively. The authors reported a prevalence of obesity of around 15–20% based on body fat percentage in participants over the age of 60 years [39]. In a study by Lii et al. in China involving 2393 older adults, obesity was found as fat percentage ≥ 25% in males and ≥ 35% in females when obesity was defined as the 60th percentile of older adults’ fat percentage. [40]. In this study, when obesity was defined as above the 60th percentile in fat percentage, the prevalence of obesity in older adults was found to be 41.8% [40]. In Turkey, Bahat et al., when defining obesity as a fat percentile above the 60th percentile, reported around 40% of older adults to be obese [13]. In the present study, daily physical activity status and the presence of depression, which is a chronic disease, were found to be associated with a risk of malnutrition in fatty obese older adults similar to the cited studies. [10, 11, 38, 39]. These important studies, however, have not been followed up by further studies investigating the frequency of malnutrition risk and the related factors in fatty obese older adults.
The strengths of our study include the wealth of data it provides on a large number of older community-dwelling adults, and its status as the first study to examine undernutrition screening in obese adults based on fat percentage in our community. The most significant strength of the present study is its use of a definition of obesity based on the physiological body composition in older adults. The limitations of the present study, on the other hand, include the unavailability of some data due to the retrospective study design, and the exclusion of people at serious risk of malnutrition, such as those with heart failure, due to the use of BIA. Another limitation is related to the lack of awareness among physicians about the term “obesity” when based on fat percentage, leading to a lack of studies in literature for comparison.
Conclusion
We confirm that also in our country, as found in previous studies [36,37,38,39,40], the prevalence of undernutrition in fatty obese older adults is high. We identified depression and low daily physical activity as related factors in undernutrition in fatty obese older adults. Although our study is important in terms of the presented results, prospective studies are needed to allow a more detailed comparison of the results.
Availability of data and material
The data is available from the authors upon reasonable request.
Code availability
N/A.
References
Cusi K, Isaacs S, Barb D et al (2022) American association of clinical endocrinology clinical practice guideline for the diagnosis and management of nonalcoholic fatty liver disease in primary care and endocrinology clinical settings: co-sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract 28:528–562
Flegal KM, Kruszon-Moran D, Carroll MD et al (2016) Trends in obesity among adults in the US, 2005 to 2014. JAMA 315:2284–2291
United Nations (2017) World population prospects: the 2017 revision, key findings and advance tables. United Nations, New York
Lynch DH, Petersen CL, Fanous MM et al (2022) The relationship between multimorbidity, obesity and functional impairment in older adults. J Am Geriatr Soc 70:1442–1449
Covinsky KE, Hilton J, Lindquist K et al (2006) Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care 44:149–157
Someya Y, Tamura Y, Kaga H et al (2022) Sarcopenic obesity is associated with cognitive impairment in community-dwelling older adults: the Bunkyo Health Study. Clin Nutr 41:1046–1051
Tsai TC, Li CI, Liu CS et al (2022) Obesity marker trajectories and cognitive impairment in older adults: a 10-year follow-up in Taichung community health study for elders. BMC Psychiatry 22:748
Dao T, Green AE, Kim YA et al (2020) Sarcopenia and muscle aging: a brief overview. Endocrinol Metab 35:716–732. https://doi.org/10.3803/EnM.2020.405
Obesity: Preventing and managing the global epidemic. Geneva 2000; [cited 2018 16.05.2018]. Available from: http://apps.who.int/iris/bitstream/10665/42330/1/WHO_TRS_894.pdf?ua1⁄41&ua1⁄41
Zoico E, Di Francesco V, Guralnik JM et al (2004) Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord 28:234–241
Bahat G, Kilic C, Topcu Y et al (2020) Fat percentage cutoff values to define obesity and prevalence of sarcopenic obesity in community-dwelling older adults in Turkey. Aging Male 23:477–482
Sanz-París A, Gomez-Candela C, Martín-Palmero A et al (2016) Application of the new ESPEN definition of malnutrition in geriatric diabetic patients during hospitalization: a multicentric study. Clin Nutr 35:1564e7
Curtis LJ, Bernier P, Jeejeebhoy K et al (2017) Costs of hospital malnutrition. Clin Nutr 36:1391e6
Cederholm T, Jensen GL (2017) To create a consensus on malnutrition diagnostic criteria: a report from the global leadership initiative on malnutrition (GLIM) meeting at the ESPEN Congress 2016. Clin Nutr 36:7e10
Cederholm T, Jensen GL (2017) To create a consensus on malnutrition diagnostic criteria. J Parenter Enteral Nutr 41:311e4
Jensen GL, Cederholm T, Correia MITD et al (2019) GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr 43:32–40
Cerri AP, Bellelli G, Mazzone A et al (2015) Sarcopenia and malnutrition in acutely ill hospitalized elderly: prevalence and outcomes. Clin Nutr 34:745e51
Koh GC-H, Chen CH, Petrella R et al (2013) Rehabilitation impact indices and their independent predictors: a systematic review. BMJ Open 3:e003483
Wakabayashi H, Sakuma K (2014) Rehabilitation nutrition for sarcopenia with disability: a combination of both rehabilitation and nutrition care management. J Cachexia Sarcopenia Muscle 5:269
Ferner RE, Aronson JK (2006) Communicating information about drug safety. BMJ 333:143–145
Delgado DA, Lambert BS, Boutris N et al (2018) Validation of digital visual analog scale pain scoring with a traditional paper-based visual analog scale in adults. J Am Acad Orthop Surg Glob Res Rev 2:e088
Katz S, Ford AB, Moskowitz RW et al (1963) Studies of illness in the aged. The index of Adl: a standardized measure of biological and psychosocial function. JAMA 185:914–919
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Cho CY, Alessi CA, Cho M et al (1998) The association between chronic illness and functional change among participants in comprehensive geriatric assessment program. J Am Geriatr Soc 46:677–682
Morley JE, Malmstrom TK, Miller DK (2012) A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 16:601–608
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol Group. Ann Med 33:337–343
Roberts HC, Denison HJ, Martin HJ et al (2011) A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 40:423–429
Wang JG, Zhang Y, Chen HE et al (2013) Comparison of two bioelectrical impedance analysis devices with dual energy X-ray absorptiometry and magnetic resonance imaging in the estimation of body composition. J Strength Cond Res 27:236–243
Kaiser MJ, Bauer JM, Ramsch C et al (2009) Validation of the mini nutritional assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 13:782–788
Bahat G, Kilic C, Altinkaynak M et al (2020) Comparison of standard versus population-specific handgrip strength cut-off points in the detection of probable sarcopenia after launch of EWGSOP2. Aging Male 23:1564–1569
Bahat G, Tufan A, Tufan F et al (2016) Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 35:1557–1563
Jésus P, Guerchet M, Pilleron S et al (2017) Undernutrition and obesity among elderly people living in two cities of developing countries: prevalence and associated factors in the EDAC study. Clin Nutr ESPEN 21:40–50
Turconi G, Rossi M, Roggi C et al (2013) Nutritional status, dietary habits, nutritional knowledge and self-care assessment in a group of older adults attending community centres in Pavia, Northern Italy. J Hum Nutr Diet 26:48–55
Kaiser MJ, Bauer JM, Uter W et al (2011) Prospective validation of the modified mini nutritional assessment short-forms in the community, nursing home, and rehabilitation setting. J Am Geriatr Soc 59:2124–2128
Bahat G, Tufan F, Saka B et al (2012) Which body mass index (BMI) is better in the elderly for functional status? Arch Gerontol Geriatr 54:78–81
Soysal P, Koc Okudur S, Kilic N et al (2022) The prevalence of undernutrition and associated factors in older obese patients. Aging Clin Exp Res 34:2023–2030
Özkaya I, Gürbüz M (2019) Malnourishment in the overweight and obese elderly. Nutr Hosp 36:39–42
Rolland Y, Lauwers-Cances V, Cristini C et al (2009) Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr 89:1895–1900
Kim TN, Yang SJ, Yoo HJ et al (2009) Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 33:885–892
Li L, Wang C, Bao Y et al (2012) Optimal body fat percentage cut-offs for obesity in Chinese adults. Clin Exp Pharmacol Physiol 39:393–398
Funding
None.
Author information
Authors and Affiliations
Contributions
MEB: writing review and editing, orijinal draft preparation, formal analysis, software, TE: writing—review and editing; NMC: writing—review and editing; SO: data curation and editing; CK: data curation, resources, investigation; GB: writing review and editing; MAK: conceptualization, methodology, supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethics approval
We obtained ethical approval from the a Local Medical School ethical board (Approval number: 2023/222). The study was conducted in compliance with the current guidelines of the Helsinki Declaration.
Statement of human and animal rights
All the procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee.
Consent to participate
We received informed consent from all participants.
Consent for publication
We received informed consent from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bozkurt, M.E., Erdogan, T., Catikkas, N.M. et al. Undernutrition in obese older adults by fat percentage. Aging Clin Exp Res 36, 3 (2024). https://doi.org/10.1007/s40520-023-02650-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40520-023-02650-1