Abstract
Objective
Anemia is one of the common adverse reactions after hip fracture surgery. The traditional method to solve anemia is allogeneic transfusion. However, the transfusion may lead to some complications such as septicemia and fever. So far, few studies have reported roles of machine learning in predicting whether blood transfusion is needed or not after hip fracture surgery. Therefore, the purpose of this study is to develop machine learning models to predict the likelihood of postoperative blood transfusion in patients undergoing hip fracture surgery.
Methods
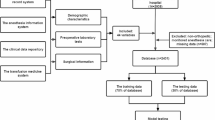
This study enrolled 1355 patients who underwent hip fracture surgery at the Affiliated Hospital of Qingdao University from January 2016 to December 2021. Among all patients, 210 cases received postoperative blood transfusion. All patients were randomly divided into a training group and a testing group at a ratio of 7:3. In the training group, univariate and multivariate logistic regression analyses were used to determine independent risk factors for the postoperative transfusion. Then, based on these independent risk factors, tenfold cross-validation method was utilized to develop five machine learning models, including logistic, multilayer perceptron (MLP), extreme gradient boosting (XGBoost), random forest (RF), and support vector machine (SVM). The receiver operating characteristic (ROC) curve, area under ROC curve (AUC), and Matthews correlation coefficient (MCC) were generated to evaluate the performance of the models. Calibration plot and decision curve analysis (DCA) were used to test the performance, stability, and clinical applicability of the models. The models were validated using the testing group; and the ROC curve, MCC, calibration plot, and DCA curves were also generated to validate the performance, stability, and clinical applicability of the models. To further verify the robustness of the model, we randomly grabbed 70% of the samples in the testing set, performed 1000 iterations, and calculated the AUC and confidence interval of the five models. Finally, we used SHapley Additive exPlanations (SHAP) to explain these models.
Results
Multivariate logistic regression analysis showed that there were 8 independent risk factors, including age, blood transfusion history, albumin (ALB), globulin (GLO), total bilirubin (TBIL), indirect bilirubin (IBIL), hemoglobin (HB), and blood loss > 200 ml. We finally selected five independent risk factors including HB, GLO, age, IBIL, and blood loss > 200 ml. Based on these five independent risk factors, we generated six characteristic variables, namely HB, HB × HB, HB × blood loss, GLO × HB, age, age × IBIL, and established five machine learning models using a tenfold cross-validation method. In the training group, the AUC values of logistic, RF, MLP, SVM, and XGB were 0.9320, 0.8911, 0.9327, 0.9225, and 0.8825, respectively, and the average AUC was 0.9122 ± 0.0212. The MCC values were 0.65, 0.77, 0.65, 0.66, and 0.68, respectively, and the calibration plot and DCA performed well. In the testing group the AUC values of logistic, RF, MLP, SVM, and XGB were 0.8483, 0.7978, 0.8576, 0.8598, and 0.8216, respectively. The average AUC was 0.8370 ± 0.0238, and the MCC values were 0.41, 0.35, 0.40, 0.41, and 0.41, respectively. The calibration plot and DCA in the testing group also showed good performance. The AUC values and confidence intervals of the 1000-iteration model were: logistic (AUC, min confidence interval [CI]–max confidence interval [CI] 0.848, 0.804–0.903), RF (AUC, minCI–maxCI 0.797, 0.734–0.857), MLP (AUC, minCI–maxCI 0.858, 0.812–0.902), SVM (AUC, minCI–maxCI 0.859, 0.819–0.910), and XGB (AUC, minCI–maxCI 0.821, 0.764–0.894). The model performed well. Finally, according to SHAP, among all five models, HB played the most important role in model prediction and interpretation.
Conclusion
The five models we developed all performed well in predicting the likelihood of blood transfusion after hip fracture surgery. Therefore, we believed that the prediction model based on machine learning had great application prospects in clinical practice, which could help clinicians better predict the risk of blood transfusion after hip fracture surgery.






Similar content being viewed by others
References
Lu X, Wang Z, Chong F et al (2022) A new nomogram model for predicting 1-year all-cause mortality after hip arthroplasty in nonagenarians with hip fractures: a 20-year period retrospective cohort study. Front Surg 9:926745. https://doi.org/10.3389/fsurg.2022.926745
Zhang C, Feng J, Wang S et al (2020) Incidence of and trends in hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med 17:e1003180. https://doi.org/10.1371/journal.pmed.1003180
Christiano AV, Elsevier HC, Sarker S et al (2021) Improving outcomes after hip fracture at a safety net hospital with a standardised hip fracture protocol. Hip Int 31:696–699. https://doi.org/10.1177/1120700020919332
Pan L, Liu Z, Wu H et al (2023) Construction and validation of a nomogram for predicting acute kidney injury after hip fracture surgery. Clin Interv Aging 18:181–191. https://doi.org/10.2147/cia.S399314
Mueller MM, Van Remoortel H, Meybohm P et al (2019) Patient blood management: recommendations from the 2018 Frankfurt consensus conference. JAMA 321:983–997. https://doi.org/10.1001/jama.2019.0554
Verlicchi F, Desalvo F, Zanotti G et al (2011) Red cell transfusion in orthopaedic surgery: a benchmark study performed combining data from different data sources. Blood Transfusion = Trasfusione del sangue 9:383–387. https://doi.org/10.2450/2011.0095-10
Wang H, Wang K, Lv B et al (2021) Establishment and assessment of a nomogram for predicting blood transfusion risk in posterior lumbar spinal fusion. J Orthop Surg Res 16:39. https://doi.org/10.1186/s13018-020-02053-2
Busch MP, Kleinman SH, Nemo GJ (2003) Current and emerging infectious risks of blood transfusions. JAMA 289:959–962. https://doi.org/10.1001/jama.289.8.959
Liu Y, Zhao S, Du W et al (2023) Applying interpretable machine learning algorithms to predict risk factors for permanent stoma in patients after TME. Front Surg 10:1125875. https://doi.org/10.3389/fsurg.2023.1125875
Chen S, Jian T, Chi C et al (2022) Machine learning-based models enhance the prediction of prostate cancer. Front Oncol 12:941349. https://doi.org/10.3389/fonc.2022.941349
Zhao H, You J, Peng Y et al (2021) Machine learning algorithm using electronic chart-derived data to predict delirium after elderly hip fracture surgeries: a retrospective case-control study. Front Surg 8:634629. https://doi.org/10.3389/fsurg.2021.634629
Huang CB, Tan K, Wu ZY et al (2022) Application of machine learning model to predict lacunar cerebral infarction in elderly patients with femoral neck fracture before surgery. BMC Geriatr 22:912. https://doi.org/10.1186/s12877-022-03631-1
Li Y, Chen M, Lv H et al (2021) A novel machine-learning algorithm for predicting mortality risk after hip fracture surgery. Injury 52:1487–1493. https://doi.org/10.1016/j.injury.2020.12.008
Lundberg SM, Lee SI (2017) A Unified Approach to Interpreting Model Predictions. In: 31st Annual Conference on Neural Information Processing Systems (NIPS): Dec 04–09 2017; Long Beach, CA. LA JOLLA: Neural Information Processing Systems (Nips)
Lundberg SM, Nair B, Vavilala MS et al (2018) Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat Biomed Eng 2:749–760. https://doi.org/10.1038/s41551-018-0304-0
Rudin C (2019) Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat Mach Intell 1:206–215. https://doi.org/10.1038/s42256-019-0048-x
Pesapane F, Codari M, Sardanelli F (2018) Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp 2:35. https://doi.org/10.1186/s41747-018-0061-6
Li W, Wang J, Liu W et al (2021) Machine learning applications for the prediction of bone cement leakage in percutaneous vertebroplasty. Front Public Health 9:812023. https://doi.org/10.3389/fpubh.2021.812023
Tseng YJ, Wang HY, Lin TW et al (2020) Development of a machine learning model for survival risk stratification of patients with advanced oral cancer. JAMA NetW Open 3:e2011768. https://doi.org/10.1001/jamanetworkopen.2020.11768
Liu Y, Song C, Tian Z et al (2023) Identification of high-risk patients for postoperative myocardial injury after CME using machine learning: a 10-year multicenter retrospective study. Int J Gen Med 16:1251–1264. https://doi.org/10.2147/ijgm.S409363
Arshi A, Lai WC, Iglesias BC et al (2021) Blood transfusion rates and predictors following geriatric hip fracture surgery. Hip Int 31:272–279. https://doi.org/10.1177/1120700019897878
Farrow L, Brasnic L, Martin C et al (2022) A nationwide study of blood transfusion in hip fracture patients : linked analysis from the Scottish hip fracture audit and the scottish national blood transfusion service. Bone Jt J 104-b:1266–1272. https://doi.org/10.1302/0301-620x.104b11.Bjj-2022-0450.R1
Liu B, Pan J, Zong H et al (2021) Establishment and verification of a perioperative blood transfusion model after posterior lumbar interbody fusion: a retrospective study based on data from a local hospital. Front Surg 8:695274. https://doi.org/10.3389/fsurg.2021.695274
Bian FC, Cheng XK, An YS (2021) Preoperative risk factors for postoperative blood transfusion after hip fracture surgery: establishment of a nomogram. J Orthop Surg Res 16:406. https://doi.org/10.1186/s13018-021-02557-5
Suh YS, Nho JH, Seo J et al (2021) Hip fracture surgery without transfusion in patients with hemoglobin less than 10 g/dL. Clin Orthop Surg 13:30–36. https://doi.org/10.4055/cios20070
Wang J, Zhao Y, Jiang B et al (2021) Development of a nomogram to predict postoperative transfusion in the elderly after intramedullary nail fixation of femoral intertrochanteric fractures. Clin Interv Aging 16:1–7. https://doi.org/10.2147/cia.S253193
Karademir G, Bilgin Y, Erşen A et al (2015) Hip fractures in patients older than 75 years old: Retrospective analysis for prognostic factors. Int J Surg 24:101–104. https://doi.org/10.1016/j.ijsu.2015.11.009
Brunskill SJ, Millette SL, Shokoohi A et al (2015) Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev 4:CD009699. https://doi.org/10.1002/14651858.CD009699.pub2
Gruson KI, Accousti KJ, Parsons BO et al (2009) Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg 18:225–230. https://doi.org/10.1016/j.jse.2008.08.005
Sun L, Liu W, Li C et al (2022) Construction and internal validation of a predictive model for risk of gastrointestinal bleeding in children with abdominal Henoch-Schönlein purpura: a single-center retrospective case-control study. Front Immunol 13:1025335. https://doi.org/10.3389/fimmu.2022.1025335
Gligorijević N, Minić S, Robajac D et al (2019) Characterisation and the effects of bilirubin binding to human fibrinogen. Int J Biol Macromol 128:74–79. https://doi.org/10.1016/j.ijbiomac.2019.01.124
Jian Y, Zhao L, Wang H et al (2020) Bilirubin: a novel predictor of hemorrhagic transformation and symptomatic intracranial hemorrhage after mechanical thrombectomy. Neurol Sci 41:903–909. https://doi.org/10.1007/s10072-019-04182-x
Eghbal MH, Samadi K, Khosravi MB et al (2019) The impact of preoperative variables on intraoperative blood loss and transfusion requirements during orthotopic liver transplant. Exp Clin Transplant 17:507–512. https://doi.org/10.6002/ect.2016.0325
Lee MH, Tsou YK, Lin CH et al (2016) Predictors of re-bleeding after endoscopic hemostasis for delayed post-endoscopic sphincterotomy bleeding. World J Gastroenterol 22:3196–3201. https://doi.org/10.3748/wjg.v22.i11.3196
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest to disclose.
Ethical approval
This study was conducted with approval from the Ethics Committee of the Affiliated Hospital of Qingdao University, with approval No. QYFY -WZLL-27908. Yes, this study was conducted in accordance with the declaration of Helsinki, and do compliance with Ethical Standards.
Human and animal rights
ll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from the participants or their guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Pan, J., Li, Y. et al. Application of machine learning model in predicting the likelihood of blood transfusion after hip fracture surgery. Aging Clin Exp Res 35, 2643–2656 (2023). https://doi.org/10.1007/s40520-023-02550-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02550-4