Abstract
Background
Nutritional status is a critical factor throughout COVID-19 disease course. Malnutrition is associated with poor outcomes in hospitalized COVID-19 patients.
Aim
To assess the prevalence of malnutrition and identify its associated factors in COVID-19 survivors.
Methods
Study cohort included 1230 COVID-19 survivors aged 18–86 attending a post-COVID-19 outpatient service. Data on clinical parameters, anthropometry, acute COVID-19 symptoms, lifestyle habits were collected through a comprehensive medical assessment. Malnutrition was assessed according to Global Leadership Initiative on Malnutrition (GLIM) criteria.
Results
Prevalence of malnutrition was 22% at 4–5 months after acute disease. Participants who were not hospitalized during acute COVID-19 showed a higher frequency of malnutrition compared to those who needed hospitalization (26% versus 19%, p < 0.01). Malnutrition was found in 25% COVID-19 survivors over 65 years of age compared to 21% younger participants (p < 0.01). After multivariable adjustment, the likelihood of being malnourished increased progressively and independently with advancing age (Odds ratio [OR] 1.02; 95% CI 1.01–1.03) and in male participants (OR 5.56; 95% CI 3.53–8.74). Malnutrition was associated with loss of appetite (OR 2.50; 95% CI 1.73–3.62), and dysgeusia (OR 4.05; 95% CI 2.30–7.21) during acute COVID-19.
Discussion
In the present investigation we showed that malnutrition was highly prevalent in a large cohort of COVID-19 survivors at 4–5 months from acute illness.
Conclusions
Our findings highlight the need to implement comprehensive nutritional assessment and therapy as an integral part of care for COVID-19 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutrition is a major health determinant which is often overlooked in infectious diseases. Malnutrition, in particular protein-energy undernutrition, is the primary cause of immunodeficiency worldwide [1]. Infection in turn may lead to weight loss and (macro/micro-) nutrient deficiencies that further perturb immune response in a self-enhancing vicious cycle [1, 2].
In COVID-19, nutritional status plays a key role across all disease stages, especially in people at high risk of developing adverse outcomes (i.e., older adults and persons with multimorbidity) [3]. Poor nutrition may increase susceptibility to SARS-Cov-2 infection [4]. In mild-to-moderate COVID-19, the most prevalent symptoms, such as fever, fatigue, loss of appetite and alterations in smell and taste, are associated with reduced food intake, weight loss and higher risk of malnutrition [5]. In hospitalized COVID-19 patients, low levels of blood nutritional biomarkers (e.g. albumin and lymphocyte counts) are linked to worse outcomes [6, 7]. Recent evidence showed that malnutrition extended hospitalization in COVID-19 patients [8, 9]. Prolonged hospital stays and intensive care unit admission are associated with substantial loss of muscle mass and strength, reduced physical function and higher risk of malnutrition [10]. Following Sars-Cov-2 infection, catabolic processes and anorexia may be aggravated by excessive inflammatory response. These phenomena may further exacerbate malnutrition and lead to impaired recovery, loss of independence, disability, and reduced quality of life after hospital discharge [11]
Prevalence of malnutrition in hospitalized COVID-19 ranges from 14 to 70% depending on the study population, intensity of care, and screening/assessment tool used [12]. In a Chinese cohort of hospitalized older adults, 27.5% were at risk of malnutrition and 52.7% malnourished according to the Mini Nutritional Assessment (MNA) [13]. In a cohort of older adults from Italy, 77% were at nutritional risk using modified Nutritional Risk Screening 2002 and approximately 50% malnourished (according to Global Leadership Initiative on Malnutrition [GLIM] criteria) [14]. In this regard, both prevalence and severity of malnutrition were greater in intensive care compared to intermediate care and rehabilitation units. Similarly, in a cohort of French COVID-19 patients hospitalized in non-intensive medical units, the overall prevalence of malnutrition was 42.1% and rose to 66.7% in patients transferred to intensive care unit [15]. Comparable results were observed in a prospective observational cohort study (NUTRI-COV) conducted in Toulouse, in which 37.5% of COVID-19 inpatients were malnourished [16]. A recent systematic review and metanalysis showed that the pooled prevalence of malnutrition among hospitalized patients with COVID-19 was 49,1% and the risk of mortality in malnourished patients was ten times higher compared to well-nourished peers [17].
A large share of COVID-19 survivors reports persistent symptoms several weeks or months after Sars-CoV-2 infection, the so-called post-COVID syndrome or Long Covid [18, 19]. In this context, malnutrition may represent both a cause and a consequence of Long Covid and a useful metric to monitor recovery from acute disease [20, 21].
The aim of the present study was to assess the prevalence of malnutrition at 4–5 months after Sars-CoV-2 infection in a large sample of COVID-19 survivors attending a dedicated outpatient service. The association of malnutrition with clinical and functional characteristics was also investigated, with a particular focus on older adults.
Materials and methods
Data for the present investigation were from the Gemelli Against COVID-19 Post-Acute Care (GAC19-PAC) project. GAC19-PAC is an initiative developed by the Department of Geriatrics, Neuroscience and Orthopedics of the Catholic University of the Sacred Heart (Rome, Italy). GAC19-PAC was conducted at a dedicated outpatient service which was established in April 2020 at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS (Rome, Italy) to investigate long-term consequences of COVID-19 and their impact on health and quality of life [19,20,21]. Details about the post-COVID-19 outpatient service and patient evaluation were described elsewhere [22]
Study Sample and data collection
The study population included adult subjects admitted to the post-COVID-19 outpatient service between April 2020 and November 2021.
Patients were offered a comprehensive medical assessment. A multidisciplinary approach was conducted to assess long-term consequences of SARS-CoV-2 infection [23] All clinical parameters, including medical history and medication inventory, lifestyle habits (e.g., smoking status and physical activity), education level and anthropometric measures were collected in a structured electronic data collection system. Smoking habit was categorized as current or never/former smoker. Regular participation in physical activity was considered as the engagement in leisure-time physical activity and/or exercise training at least twice weekly during the past year [24].
Both acute COVID-19 and persistent symptoms were collected on admission using a standardized questionnaire, as previously described [18].
COVID-19 severity was categorized as follows: (a) no hospitalization; (b) hospitalization not requiring supplemental oxygen; (c) hospitalization with oxygen supplementation; (d) hospitalization with high-flow oxygen supplementation; (e) hospitalization with intensive care unit (ICU) admission with invasive ventilation [25]
Time from COVID-19 diagnosis to study inclusion was calculated based on self-report.
Nutritional assessment
Body weight was measured through an analog medical scale. Body height was measured using a standard stadiometer. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters). Low BMI was defined as having a BMI below 20 kg/m2 in subjects younger than 70 years of age and below 22 kg/m2 if 70 years and older.
Body composition was measured in a fasting state using direct segmental multifrequency bioelectrical impedance (BIA) equipment (InBody S10, Seoul, Korea). Appendicular skeletal mass (SM) index was calculated using validated BIA prediction equation (SM/height2) and expressed in kg/m2 [26, 27]. According to the European Working Group on Sarcopenia in Older People (EWGSOP) [28, 29], reduced muscle mass was identified as an appendicular skeletal mass index below 8.87 kg/m2 in males and 6.42 kg/m2 in females [27].
Data on appetite and dietary intake of study participants during acute COVID-19 were collected through a face-to-face interview by a registered dietitian.
Finally, according to the Global Leadership Initiative on Malnutrition (GLIM) criteria [30], participants were diagnosed as malnourished if they met at least one phenotypic criterion (low BMI and/or reduced muscle mass) and at least one etiologic criterion (reduced food intake and/or inflammation) (Table 1). The presence of inflammation was defined as having C-Reactive Protein (CRP) levels above 5 mg/L.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation (SD), categorical variables as frequencies by absolute value and percentage (%) of the total. Descriptive statistics were used to report clinical characteristics of the study population according to malnutrition status. The differences in proportions and means of covariates between study participants with and without malnutrition were assessed using Fisher’s Exact Test and t test statistics, respectively.
Logistic regression models were built to assess the association between clinical and functional characteristics and malnutrition status. Candidate variables to be included in the regression models were selected based on biological and clinical plausibility. To identify factors independently associated with malnutrition, we first estimated crude odds ratio (OR) and its 95% confidence interval (CI). A multivariable regression model was computed including all the variables that were associated with the outcome at α level of 0.05, after adjustment for age and gender. Model 1 included all clinical variables and COVID-19 symptoms potentially related with malnutrition. Model 2 was built adding COVID-19 severity scores.
All analyses were performed using SPSS software (version 11.0, SPSS Inc., Chicago, IL).
Results
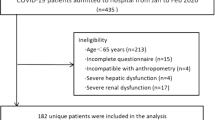
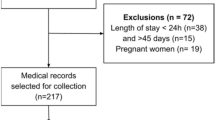
Between April 2020 and November 2021, 2100 COVID-19 survivors were admitted to the post-COVID-19 outpatient service. The present investigation included 1230 participants (mean age 54.6 ± 14.1 years; age range 18–86; 48% women) for whom all variables of interest were available.
The prevalence of malnutrition identified according to GLIM criteria was 22%, with no significant difference between males and females (23% vs 21%, p = 0.28). The average time from COVID-19 diagnosis to the inclusion in the study was significantly higher among participants with malnutrition versus non-malnourished peers (147±81 versus 131 ± 70, p = 0.01). Figure 1 shows the prevalence of malnutrition according to hospitalization and age groups. Interestingly, at the time of admission at post-COVID-19 outpatient service, participants who were not hospitalized during acute COVID-19 showed a higher rate of malnutrition compared to those who needed hospitalization (26% versus 19%, p < 0.01). Malnutrition was diagnosed in 25% of COVID-19 survivors older than 65 years, compared to 21% of younger participants (p < 0.01). In particular, in older participants, the prevalence of malnutrition was significantly higher in males compared to females (30% versus 17%, p < 0.001).
Characteristics of the study population according to malnutrition status are summarized in Table 2. Participants with malnutrition had lower BMI than those who were well-nourished (22.5 ± 3.5 versus 27.2 ± 4.7, p < 0.001). No differences in comorbidities and hematological parameters were observed. Notably, the prevalence of specific COVID-19-related symptoms during acute disease (loss of appetite, dysgeusia, myalgia, joint pain, smell disorders, headache and skin lesion) was significantly higher among malnourished participants compared to those who were not malnourished (Table 3).
Logistic regression models were used to evaluate the association between clinical and functional characteristics during acute COVID and malnutrition. After multivariable adjustment (Model 2), the likelihood of being malnourished increased progressively and independently with advancing age (odds ratio [OR] 1.02; 95% CI 1.01–1.03) and the risk was significantly higher among male participants (OR 5.56; 95% CI 3.53–8.74). Malnutrition at the time of the study visit was associated with two symptoms reported by participants during the acute phase of COVID-19: loss of appetite (OR 2.50; 95% CI 1.73–3.62), and dysgeusia (OR 4.05; 95% CI 2.30–7.21) (Table 4).
In the post-acute phase, the most frequently reported symptoms were fatigue (65%), dyspnea (60%) and joint pain (42%). A gender-specific distribution of persistent symptoms was observed. Female participants showed a consistently higher prevalence of COVID19-related symptoms than males, regardless of malnutrition status. However, male participants with malnutrition reported a higher prevalence of anosmia, dysgeusia and lack of appetite than non-malnourished persons, while these differences were not present among female participants (Fig. 2).
Discussion
In the present study, we explored the prevalence of malnutrition (according to GLIM criteria [31]) in a large sample of COVID-19 survivors at 4–5 months after acute disease. Several studies focused on the nutritional status of COVID 19 patients during hospital stay or after discharge [12]. However, to our knowledge, this is the first investigation to include a large sample of non-hospitalized COVID-19 survivors. Another important contribution from our study is the large number of participants included, although from a single center.
A wide range of malnutrition prevalence (14–70%) was reported in hospitalized COVID-19 patients during acute disease [12], with the majority being estimated at around 40% [17, 32].
Overall, the prevalence of malnutrition observed in our study sample was 22%, which indicates that malnutrition was present in a large share of COVID-19 survivors months after acute illness and may hinder full recovery in people with Long Covid.
Our findings are in keeping with recent evidence from other post-COVID outpatient services which showed that malnutrition is one of the most common conditions among COVID-19 survivors [33]. At 23 days after hospital discharge, 54.7% of patients were at risk of malnutrition and 6.6% malnourished in a prospective observational study of 213 patients at a single center in Italy [5]. In a longitudinal study conducted on 91 hospitalized patients, 28.6% were malnourished at 30 days post-discharge, compared to 42.3% at admission [34]. In this cohort, the most relevant predictors of malnutrition were the need for high-flow oxygen therapy and/or invasive ventilation during hospitalization. In a cohort of 92 subjects attending a post-COVID recovery clinic at 3 months after Sars-CoV-2 infection, approximately half were at risk of malnutrition, which was associated with persistent gastrointestinal symptoms and reduced energy and protein intake [35]. In a French prospective cohort study on 288 hospitalized COVID-19 patients, 56.9% presented malnutrition at discharge according to GLIM criteria [36]. Of them, 47.2% showed persistent malnutrition at 30 days, and 36% at six months [20]. The most relevant factors for persistent malnutrition were intensive care unit admission and obesity.
In our study population, malnutrition risk increased with age, male gender, and was associated with loss of appetite and dysgeusia. Smell and taste dysfunction, and lack of appetite are highly prevalent symptoms related to COVID-19 that may have an impact on quality of life and overall health long after Sars-Cov-2 infection [18, 19, 37]. Poor taste is associated with lack of appetite and reduced dietary quality (low protein and high fat intake) in older adults [38].
Notably, poor appetite, and smell and taste disorders are traditionally associated with anorexia of aging, a major contributor to undernutrition and adverse health outcomes in older adults [39]. This corroborates the hypothesis that mechanisms involved in Long Covid may overlap with those of the aging process and aggravate pre-existing degenerative conditions [40, 41]. The strong association between poor appetite, dysgeusia and malnutrition risk found in our investigation underlines the need for a comprehensive nutritional assessment in COVID-19 patients throughout the disease course. In this regard, major clinical nutrition societies strongly recommend accurate and timely nutritional assessment and intervention to improve clinical outcomes in people at risk of malnutrition, especially in older adults, and in persons with multimorbidity [11, 42, 43]. Recently, European Society for Clinical Nutrition and Metabolism (ESPEN) developed a practical guidance for nutritional management of individuals with SARS-CoV-2 infection [44]. According to ESPEN recommendations, prevention, diagnosis and treatment of malnutrition should be considered as an integral part of COVID-19 patients’ continuum of care [44]. In this context, preliminary data from our post-COVID outpatient service showed that a comprehensive nutritional assessment together with the use of nutritional supplements (e.g., essential amino acids and derivatives) had a positive effects on nutritional status, functional recovery, and quality of life in COVID-19 survivors [45,46,47].
A sex-dependent distribution of acute and post-acute symptoms of COVID-19 was often observed. While a higher prevalence of acute symptoms (along with higher disease severity and mortality) was widely found in men, post-acute symptoms were more frequently reported by women [48, 49]. Our results are in line with recent systematic reviews and meta-analyses that have shown a sex-specific distribution of persisting symptoms, with more symptoms reported by women [48, 50]. However, in the present investigation, only male participants showed significant differences in the persistence of COVID19-related symptoms according to malnutrition status during the post-acute phase (Fig. 2). Specifically, anosmia, dysgeusia and inappetence were greater among malnourished participants, suggesting an enduring contribution of these symptoms to malnutrition status at all phases of the disease.
Limitations of the study include the lack of information on nutritional status before and at the time of acute COVID-19, and the lack of details on malnutrition severity. Moreover, this is a single center study with a large number of patients but without a control group (e.g., patients recovering from other infectious diseases). Patients with community-acquired pneumonia or patients with other viral diseases–such as herpes or chickenpox–can also have high rate of malnutrition, suggesting these findings could be not unique to COVID-19. However, the comprehensive medical assessment conducted during the study visit excluded the presence of any other acute infection.
Conclusion
In the present study we showed that malnutrition was highly prevalent in a large cohort of COVID-19 survivors at 4–5 months from acute illness. Advanced age, poor appetite and taste disorders were the most relevant risk factors associated with malnutrition. Our findings highlight the need to implement comprehensive nutritional assessment and therapy as an integral part of COVID-19 patients’ care to optimize recovery from Sars-CoV-2 infection.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of the research participants.
References
Katona P, Katona-Apte J (2008) The interaction between nutrition and infection. Clin Infect Dis 46:1582–1588
Bourke CD, Berkley JA, Prendergast AJ (2016) Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol 37:386–398
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062
James PT, Ali Z, Armitage AE et al (2021) The role of nutrition in COVID-19 susceptibility and severity of disease: a systematic review. J Nutr 151:1854–1878
di Filippo L, de Lorenzo R, D’Amico M et al (2021) COVID-19 is associated with clinically significant weight loss and risk of malnutrition, independent of hospitalisation: a post-hoc analysis of a prospective cohort study. Clin Nutr 40:2420–2426
Wu C, Chen X, Cai Y et al (2019) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan China. JAMA Intern Med 180:934–943
Liu W, Tao ZW, Wang L et al (2020) Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 133:1032–1038
Larrazabal RB, Chiu HHC, Palileo-Villanueva LAM (2021) Outcomes of nutritionally at-risk Coronavirus Disease 2019 (COVID 19) patients admitted in a tertiary government hospital: a follow-up study of the MalnutriCoV study. Clin Nutr ESPEN 43:239–244
Yu Y, Ye J, Chen M et al (2021) Malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis. J Nutr Health Aging 25:369–73
Alvarez J, Bernal M, Serrano C et al (2021) Malnutrition, sarcopenia and disability in critical COVID 19 patients. Clin Nutr ESPEN 46:S764
Singer P, Blaser AR, Berger MM et al (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38:48–79
Thomas S, Alexander C, Cassady BA (2021) Nutrition risk prevalence and nutrition care recommendations for hospitalized and critically-ill patients with COVID-19. Clin Nutr ESPEN 44:38–49
Li T, Zhang Y, Gong C et al (2020) Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr 74:871–875
Pironi L, Sasdelli AS, Ravaioli F et al (2021) Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr 40:1330–1337
Bedock D, Bel Lassen P, Mathian A et al (2020) Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr ESPEN 40:214–219
Rouget A, Vardon-Bounes F, Lorber P et al (2021) Prevalence of malnutrition in coronavirus disease 19: the NUTRICOV study. Br J Nutr 126:1296–1303
Abate SM, Chekole YA, Estifanos MB et al (2021) Prevalence and outcomes of malnutrition among hospitalized COVID-19 patients: a systematic review and meta-analysis. Clin Nutr ESPEN 43:174–183
Carfì A, Bernabei R, Landi F (2020) Persistent symptoms in patients after acute COVID-19. JAMA 324:603–605
Tosato M, Carfì A, Martis I et al (2021) Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc 22:1840–1844
Gérard M, Mahmutovic M, Malgras A et al (2021) Long-term evolution of malnutrition and loss of muscle strength after COVID-19: a major and neglected component of long COVID-19. Nutrients 13:3964
Levy D, Giannini M, Oulehri W et al (2022) Long term follow-up of sarcopenia and malnutrition after hospitalization for COVID-19 in conventional or intensive care units. Nutrients 14:912
Landi F, Gremese E, Bernabei R et al (2020) Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res 32:1613–1620
Landi F, Barillaro C, Bellieni A et al (2020) The new challenge of geriatrics: saving frail older people from the SARS-COV-2 pandemic infection. J Nutr Health Aging 24:466–470
Landi F, Calvani R, Picca A et al (2018) Impact of habitual physical activity and type of exercise on physical performance across ages in community-living people. PLoS One 13:e0191820
Galluzzo V, Ciciarello F, Tosato M et al (2022) Association between vitamin D status and physical performance in COVID-19 survivors: results from the Gemelli against COVID-19 post-acute care project. Mech Ageing Dev 205:111684
Janssen I, Heymsfield S, Baumgartner R et al (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471
Chien M, Huang T, Wu Y (2008) Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc 56:1710–1715
Cruz-Jentoft A, Baeyens J, Bauer J et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Cruz-Jentoft A, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–313
Cederholm T, Jensen G, Correia M et al (2019) Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. Clin Nutr 38:1–9
Cederholm T, Jensen GL, Correia MITD et al (2019) GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr 38:1–9
Damayanthi HDWT, Prabani KIP (2021) Nutritional determinants and COVID-19 outcomes of older patients with COVID-19: a systematic review. Arch Gerontol Geriatr 95:104411
Grund S, Bauer JM (2022) Malnutrition and sarcopenia in COVID-19 survivors. Clin Geriatr Med 38:559
Bedock D, Couffignal J, Bel Lassen P et al (2021) Evolution of nutritional status after early nutritional management in COVID-19 hospitalized patients. Nutrients 13:2276
Deer RR, Hosein E, Harvey M et al (2022) Impact of COVID-19 infection and persistent lingering symptoms on patient reported indicators of nutritional risk and malnutrition. Nutrients 14:642
Quilliot D, Gérard M, Bonsack O et al (2021) Impact of severe SARS-CoV-2 infection on nutritional status and subjective functional loss in a prospective cohort of COVID-19 survivors. BMJ Open 11:e048948
Tan BKJ, Han R, Zhao JJ et al (2022) Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: meta-analysis with parametric cure modelling of recovery curves. BMJ 378:e069503
Fluitman KS, Hesp AC, Kaihatu RF et al (2021) Poor taste and smell are associated with poor appetite, macronutrient intake, and dietary quality but not with undernutrition in older adults. J Nutr 151:605–614
Landi F, Picca A, Calvani R et al (2017) Anorexia of aging: assessment and management. Clin Geriatr Med 33:315–323
Douaud G, Lee S, Alfaro-Almagro F et al (2022) SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604:697–707
Montes-Ibarra M, Oliveira CLP, Orsso CE et al (2022) The impact of long covid-19 on muscle health. Clin Geriatr Med 38:545
Gomes F, Schuetz P, Bounoure L et al (2018) ESPEN guidelines on nutritional support for polymorbid internal medicine patients. Clin Nutr 37:336–353
Volkert D, Beck AM, Cederholm T et al (2019) ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 38:10–47
Barazzoni R, Bischoff SC, Breda J et al (2020) ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr 39:1631–1638
Landi F, Martone AM, Ciciarello GV et al (2022) Effects of a new multicomponent nutritional supplement on muscle mass and physical performance in adult and old patients recovered from COVID-19: a pilot observational case-control study. Nutrients 14:2316
Tosato M, Ciciarello F, Zazzara MB et al (2022) Nutraceuticals and dietary supplements for older adults with long COVID-19. Clin Geriatr Med 38:565
Tosato M, Calvani R, Picca A et al (2022) Effects of l-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: a single-blind randomized controlled trial. Nutrients 14:4984
Di Gennaro F, Belati A, Tulone O et al (2022) Incidence of long COVID-19 in people with previous SARS-Cov2 infection: a systematic review and meta-analysis of 120,970 patients. Intern Emerg Med 30:1
Michelen M, Manoharan L, Elkheir N et al (2021) Characterising long COVID: a living systematic review. BMJ Glob Health 6:e005427
de Las F, Penas C, Martin Guerrero J et al (2022) Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: the LONG-COVID-EXP-CM multicenter study. J Clin Med 11:413
Acknowledgements
The Gemelli Against COVID-19 Post-Acute Care team thanks La Torre R, Brisetti S, Fella L, Sofo MT and all the nurse staff of the “Post-COVID Day Hospital Unit” for their extraordinary dedication and expertise in treating COVID-19 patients. The Gemelli Against COVID-19 Post-Acute Care Study Group is composed as follows: Steering committee: Francesco Landi, Elisa Gremese, Roberto Bernabei, Massimo Fantoni, Antonio Gasbarrini, Matteo Tosato, Carlo Romano Settanni, Serena Porcari, Francesca Benvenuto, Giulia Bramato, Vincenzo Brandi, Angelo Carfì, Francesca Ciciarello, Maria Rita Lo Monaco, Anna Maria Martone, Emanuele Marzetti, Carmen Napolitano, Vincenzo Galluzzo, Francesco Pagano, Cristina Pais, Sara Rocchi, Elisabetta Rota, Andrea Salerno, Matteo Tosato, Marcello Tritto, Riccardo Calvani, Maria Beatrice Zazzara, Lucio Catalano, Anna Picca, Giulia Savera, Mariaelena D’Elia, Damiano Biscotti, Roberto Cauda, Rita Murri, Antonella Cingolani, Giulio Ventura, Eleonora Taddei, Davide Moschese, Arturo Ciccullo, Massimo Fantoni, Leonardo Stella, Giovanni Addolorato, Francesco Franceschi, Gertrude Mingrone, Maria Assunta Zocco, Maurizio Sanguinetti, Paola Cattani, Simona Marchetti, Brunella Posteraro, Michela Sali, Alessandra Bizzarro, Alessandra Lauria, Stanislao Rizzo, Maria Cristina Savastano , Gloria Gambini, Grazia Maria Cozzupoli, Carola Culiersi, Giulio Cesare Passali, Gaetano Paludetti, Jacopo Galli, Fabrizio Crudo, Giovanni Di Cintio, Ylenia Longobardi, Laura Tricarico, Mariaconsiglia Santantonio, Tiziana Di Cesare, Mariateresa Guarino, Marco Corbò, Stefano Settimi, Dario Mele, Francesca Brigato, Danilo Buonsenso, Piero Valentini, Dario Sinatti, Gabriella De Rose, Luca Richeldi, Francesco Lombardi, Angelo Calabrese, Francesco Varone, Paolo Maria Leone, Matteo Siciliano, Giuseppe Maria Corbo , Giuliano Montemurro, Mariarosaria Calvello, Enrica Intini, Jacopo Simonetti, Giuliana Pasciuto, Veronica Adiletta, Carmelo Sofia, Maria Angela Licata, Gabriele Sani, Delfina Janiri, Alessio Simonetti, Marco Modica, Montanari Silvia, Antonello Catinari, Beatrice Terenzi, Luigi Natale, Anna Rita Larici, Riccardo Marano, Tommaso Pirronti, Amato Infante, Annamaria Paglionico, Luca Petricca, Barbara Tolusso, Stefano Alivernini, Clara Di Mario, Angelo Santoliquido, Luca Santoro, Antonio Nesci, Angela Di Giorgio, Alessia D’Alessandro.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. This study was supported by Abbott Nutrition. The study was also supported by the Italian Ministry of Health—Ricerca Corrente 2023.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MT, RC, FL, VG, AMM, BZ, CP, FC and GS The first draft of the manuscript was written by MT, RC, FL, MCR and MR. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
None of the participants in the Gemelli Against COVID-19 Post-Acute Care Study Group has any conflict of interest. MCR and MR are employees of Abbott Nutrition.
Statement of human and animal rights
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tosato, M., Calvani, R., Ciciarello, F. et al. Malnutrition in COVID-19 survivors: prevalence and risk factors. Aging Clin Exp Res 35, 2257–2265 (2023). https://doi.org/10.1007/s40520-023-02526-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02526-4