Abstract
Background
Frailty is common in Parkinson’s disease (PD) and increases vulnerability to adverse outcomes. Early detection of this syndrome aids in early intervention.
Aims
To objectively identify frailty at an early stage during routine motor tasks in PD patients using a Kinect-based system.
Methods
PD patients were recruited and assessed with the Fried criteria to determine their frailty status. Each participant was recorded performing the Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) extremity tasks with a Kinect-based system. Statistically significant kinematic parameters were selected to discriminate the pre-frail from the non-frail group.
Results
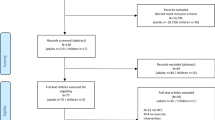
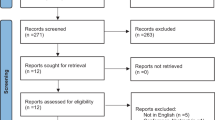
Of the fifty-two participants, twenty were non-frail and thirty-two were pre-frail. Decreased frequency in finger tapping (P = 0.005), hand grasping (P = 0.002), toe tapping (P = 0.002), and leg agility (P = 0.019) alongside reduced hand grasping speed (P = 0.030), lifting (P < 0.001) and falling speed (P < 0.001) in leg agility were observed in the pre-frail group. Amplitude in leg agility (P = 0.048) and amplitude decrement rate (P = 0.046) in hand grasping showed marginally significant differences between two groups. Moderate discriminative values were found in frequency and speed of the extremity tasks to identify pre-frailty with sensitivity, specificity, and area under the curve (AUC) in the range of 45.00–85.00%, 68.75–100%, and 0.701–0.836, respectively. The combination of frequency and speed in extremity tasks showed moderate to high discriminatory ability, with AUC of 0.775 (95% CI 0.637–0.913, P < 0.001) for upper limb tasks and 0.909 (95% CI 0.832–0.987, P < 0.001) for lower limb tasks. When combining these features in both upper and lower limb tasks, the AUC increased to 0.942 (95% CI 0.886–0.999, P < 0.001).
Conclusions
Our findings demonstrated the promise of utilizing Kinect-based kinematic data from MDS-UPDRS III tasks as early indicators of frailty in PD patients.

Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Macerollo A, Chen J-C (2016) Trends in the incidence of Parkinson disease. JAMA Neurol 73:1497–1498
Tenison E, Henderson EJ (2020) Multimorbidity and frailty: tackling complexity in Parkinson’s disease. J Parkinsons Dis 10:S85–S91
Ebina J, Ebihara S, Kano O (2022) Similarities, differences and overlaps between frailty and Parkinson’s disease. Geriatr Gerontol Int 22:259–270
Belvisi D, Canevelli M, Costanzo M et al (2022) The role of frailty in Parkinson’s disease: a cross-sectional study. J Neurol 269:3006–3014
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancet 381:752–762
Mcmillan JM, Michalchuk Q, Goodarzi Z (2021) Frailty in Parkinson’s disease: a systematic review and meta-analysis. Clin Park Relat Disord 4:100095
Collard RM, Boter H, Schoevers RA et al (2012) Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60:1487–1492
Borda MG, Pérez-Zepeda MU, Jaramillo-Jimenez A et al (2022) Frailty in Parkinson’s disease and its association with early dementia: a longitudinal study. Parkinsonism Relat Disord 99:51–57
Dent E, Martin FC, Bergman H et al (2019) Management of frailty: opportunities, challenges, and future directions. Lancet 394:1376–1386
Lang P-O, Michel J-P, Zekry D (2009) Frailty syndrome: a transitional state in a dynamic process. Gerontology 55:539–549
Sezgin D, O’donovan M, Woo J, et al (2022) Early identification of frailty: Developing an international delphi consensus on pre-frailty. Arch Gerontol Geriatr 99:104586
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Rodriguez-Mañas L, Fried LP (2015) Frailty in the clinical scenario. Lancet 385:e7–e9
Monje MHG, Foffani G, Obeso J et al (2019) New sensor and wearable technologies to aid in the diagnosis and treatment monitoring of Parkinson’s disease. Annu Rev Biomed Eng 21:111–143
Dasenbrock L, Heinks A, Schwenk M et al (2016) Technology-based measurements for screening, monitoring and preventing frailty. Z Gerontol Geriatr 49:581–595
Zhong R, Rau P-LP, Yan X (2018) Application of smart bracelet to monitor frailty-related gait parameters of older Chinese adults: a preliminary study. Geriatr Gerontol Int 18:1366–1371
Rahemi H, Nguyen H, Lee H et al (2018) Toward smart footwear to track frailty phenotypes-using propulsion performance to determine frailty. Sensors (Basel) 18:1763
Schwenk M, Mohler J, Wendel C et al (2015) Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology 61:258–267
Hong R, Zhang T, Zhang Z et al (2022) A summary index derived from Kinect to evaluate postural abnormalities severity in Parkinson’s Disease patients. NPJ Parkinsons Dis 8:96
Han J, Shao L, Xu D et al (2013) Enhanced computer vision with Microsoft Kinect sensor: a review. IEEE Trans Cybern 43:1318–1334
Akbari G, Nikkhoo M, Wang L et al (2021) Frailty level classification of the community elderly using microsoft kinect-based skeleton pose: a machine learning approach. Sensors (Basel) 21:4017
Goetz CG, Tilley BC, Shaftman SR et al (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Buongiorno D, Bortone I, Cascarano GD et al (2019) A low-cost vision system based on the analysis of motor features for recognition and severity rating of Parkinson’s Disease. BMC Med Inform Decis Mak 19:243
Ferraris C, Nerino R, Chimienti A et al (2018) A self-managed system for automated assessment of UPDRS upper limb tasks in Parkinson’s disease. Sensors (Basel) 18:3523
Wu Z, Gu H, Hong R et al (2023) Kinect-based objective evaluation of bradykinesia in patients with Parkinson’s disease. Digit Health 9:20552076231176652
Bologna M, Paparella G, Fasano A et al (2020) Evolving concepts on bradykinesia. Brain 143:727–750
Berardelli A, Rothwell JC, Thompson PD et al (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Buchman AS, Leurgans SE, Boyle PA et al (2011) Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med 9:42
Lin W-C, Huang Y-C, Leong C-P et al (2019) Associations between cognitive functions and physical frailty in patients with Parkinson’s disease. Front Aging Neurosci 11:283
Peball M, Mahlknecht P, Werkmann M et al (2019) Prevalence and associated factors of sarcopenia and frailty in Parkinson’s disease: a cross-sectional study. Gerontology 65:216–228
Ahmed NN, Sherman SJ, Vanwyck D (2008) Frailty in Parkinson’s disease and its clinical implications. Parkinsonism Relat Disord 14:334–337
Bologna M, Leodori G, Stirpe P et al (2016) Bradykinesia in early and advanced Parkinson’s disease. J Neurol Sci 369:286–291
Shin JH, Ong JN, Kim R et al (2020) Objective measurement of limb bradykinesia using a marker-less tracking algorithm with 2D-video in PD patients. Parkinsonism Relat Disord 81:129–135
Wilkins KB, Petrucci MN, Kehnemouyi Y et al (2022) Quantitative digitography measures motor symptoms and disease progression in Parkinson’s disease. J Parkinsons Dis 12:1979–1990
Fairhall N, Langron C, Sherrington C et al (2011) Treating frailty–a practical guide. BMC Med 9:83
Seiffert P, Derejczyk J, Kawa J et al (2017) Frailty phenotype and the role of levodopa challenge test in geriatric inpatients with mild parkinsonian signs. Biogerontology 18:641–650
Espay AJ, Giuffrida JP, Chen R et al (2011) Differential response of speed, amplitude, and rhythm to dopaminergic medications in Parkinson’s disease. Mov Disord 26:2504–2508
Espay AJ, Beaton DE, Morgante F et al (2009) Impairments of speed and amplitude of movement in Parkinson’s disease: a pilot study. Mov Disord 24:1001–1008
Overstall PW, Clarke CE (2002) Uncertainties in the pharmacotherapy of Parkinson’s disease and how to solve them. Gerontology 48:30–33
Minici D, Cola G, Giordano A et al (2022) Towards automated assessment of frailty status using a wrist-worn device. IEEE J Biomed Health Inform 26:1013–1022
Jung D, Kim J, Kim M et al (2021) Frailty assessment using temporal gait characteristics and a long short-term memory network. IEEE J Biomed Health Inform 25:3649–3658
Zhou H, Razjouyan J, Halder D et al (2019) Instrumented trail-making task: application of wearable sensor to determine physical frailty phenotypes. Gerontology 65:186–197
Razjouyan J, Naik AD, Horstman MJ et al (2018) Wearable sensors and the assessment of frailty among vulnerable older adults: an observational cohort study. Sensors (Basel) 18:1336
Millor N, Lecumberri P, Gomez M et al (2017) gait velocity and chair sit-stand-sit performance improves current frailty-status identification. IEEE Trans Neural Syst Rehabil Eng 25:2018–2025
Fried LP, Xue Q-L, Cappola AR et al (2009) Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 64:1049–1057
Lippi L, D’abrosca F, Folli A et al (2022) Closing the gap between inpatient and outpatient settings: integrating pulmonary rehabilitation and technological advances in the comprehensive management of frail patients. Int J Environ Res Public Health 19:9150
Coleman EA (2002) Challenges of systems of care for frail older persons: the United States of America experience. Aging Clin Exp Res 14:233–238
Schapira M, Outumuro MB, Giber F et al (2022) Geriatric co-management and interdisciplinary transitional care reduced hospital readmissions in frail older patients in Argentina: results from a randomized controlled trial. Aging Clin Exp Res 34:85–93
Kim M, Won CW (2022) Cut points of chair stand test for poor physical function and its association with adverse health outcomes in community-dwelling older adults: a cross-sectional and longitudinal study. J Am Med Dir Assoc 23:1375-1382.e3
O’hoskiBeanMa SJFJ et al (2020) Physical function and frailty for predicting adverse outcomes in older primary care patients. Arch Phys Med Rehabil 101:592–598
Arjunan A, Peel NM, Hubbard RE (2019) Gait speed and frailty status in relation to adverse outcomes in geriatric rehabilitation. Arch Phys Med Rehabil 100:859–864
Chiang P-L, Chen Y-S, Lin AW (2019) Altered body composition of psoas and thigh muscles in relation to frailty and severity of Parkinson's disease. Int J Environ Res Public Health 16:3667
Harris-Love MO, Benson K, Leasure E et al (2018) The influence of upper and lower extremity strength on performance-based sarcopenia assessment tests. J Funct Morphol Kinesiol 3:53
Beier F, Löffler M, Nees F et al (2022) Sensory and motor correlates of frailty: dissociation between frailty phenotype and frailty index. BMC Geriatr 22:755
Toosizadeh N, Mohler J, Najafi B (2015) Assessing upper extremity motion: an innovative method to identify frailty. J Am Geriatr Soc 63:1181–1186
Funding
This study was supported by National Natural Science Foundation of China (Grant number. 81974198).
Author information
Authors and Affiliations
Contributions
QG and LJ conceived and designed the study. LX wrote the manuscript. LX and RH performed the research, collected and analyzed data. ZW and RH revised the manuscript. QG and XW recruited subjects. LY, KP, SL, and JZ helped in data collection and analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The Ethics Committee of Shanghai Tongji Hospital approved all the procedures. Written informed consent was obtained from all the participants.
Human and animal rights
The study was performed in accordance with the Delaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, L., Hong, R., Wu, Z. et al. Kinect-based objective assessment for early frailty identification in patients with Parkinson’s disease. Aging Clin Exp Res 35, 2507–2516 (2023). https://doi.org/10.1007/s40520-023-02525-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02525-5