Abstract
Introduction
Peripheral neuropathies are a prevalent, heterogeneous group of diseases of the peripheral nervous system. Symptoms are often debilitating, difficult to treat, and usually become chronic. Not only do they diminish patients’ quality of life, but they can also affect medical therapy and lead to complications. To date, for most conditions there are no evidence-based causal treatment options available. Research has increased considerably since the last review in 2014 regarding the therapeutic potential of exercise interventions for patients with polyneuropathy.
Objective
Our objective in this systematic review with meta-analysis was to analyze exercise interventions for neuropathic patients in order to update a systematic review from 2014 and to evaluate the potential benefits of exercise on neuropathies of different origin that can then be translated into practice.
Methods
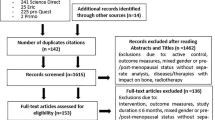
Two independent reviewers performed a systematic review with meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Inclusion criteria according to the PICOS approach were: neuropathic patients, exercise interventions only, an inactive or non-exercising control group, and solely randomized controlled trials with the following outcome parameters: neuropathic symptoms, balance parameters, functional mobility, gait, health-related quality of life, and HbA1c (glycated hemoglobin).
Results
A total of 41 randomized, controlled trials met all inclusion criteria, 20 of which could be included in the quantitative analysis. Study quality varied from moderate to high. Current data further support the hypothesis that exercise is beneficial for neuropathic patients. This is best documented for patients with diabetic peripheral neuropathy (DPN) (27 studies) as well as for chemotherapy-induced peripheral neuropathy (CIPN) (nine studies), while there are only few studies (five) on all other causes of neuropathy. We found standardized mean differences in favor of the exercise group of 0.27–2.00 for static balance, Berg Balance Scale, Timed-up-and-go-test, nerve conduction velocity of peroneal and sural nerve as well as for HbA1c in patients with DPN, and standardized mean differences of 0.43–0.75 for static balance, quality of life, and neuropathy-induced symptoms in patients with CIPN.
Conclusion
For DPN, evidence-based recommendations can now be made, suggesting a combination of endurance and sensorimotor training to be most beneficial. For patients with CIPN, sensorimotor training remains the most crucial component. For all other neuropathies, more high-quality research is needed to derive evidence-based recommendations. Overall, it seems that sensorimotor training has great potential to target most neuropathies and combined with endurance training is therefore currently the best treatment option for neuropathies.
Registration Number
(PROSPERO 2019 CRD42019124583)/16.04.2019.



Similar content being viewed by others
Notes
It should be noted that sensorimotor training is the general definition of any exercise that optimizes the interaction of the sensory and motor system to improve motor control, while balance training applies to the most commonly applied sub-category of sensorimotor training. It is used to describe the maintenance of postural control on an unstable surface or position. These two terms are frequently used interchangeably and synonymously by authors [41], which causes a diffuse simplification. Since not all studies described exactly which exercises they performed, we will be using the more general term throughout this article.
References
Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44:1289–304.
Singer MA, Vernino SA, Wolfe GI. Idiopathic neuropathy: new paradigms, new promise. J Peripher Nerv Syst. 2012;17(Suppl 2):43–9.
Lehmann HC, Wunderlich G, Fink GR, Sommer C. Diagnosis of peripheral neuropathy. Neurol Res Pract. 2020;2:20.
Mold JW, Vesely SK, Keyl BA, et al. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–18.
Visser NA, Notermans NC, Linssen RS, et al. Incidence of polyneuropathy in Utrecht, the Netherlands. Neurology. 2015;84:259–64.
Sommer C, Geber C, Young P, et al. Polyneuropathies. Dtsch Arztebl Int. 2018;115:83–90.
Tofthagen CS, Cheville AL, Loprinzi CL. The physical consequences of chemotherapy-induced peripheral neuropathy. Curr Oncol Rep. 2020;22:50.
Hoffman EM, Staff NP, Robb JM, et al. Impairments and comorbidities of polyneuropathy revealed by population-based analyses. Neurology. 2015;84:1644–51.
Stubblefield MD, Burstein HJ, Burton AW, et al. NCCN task force report: management of neuropathy in cancer. J Natl Compr Canc Netw. 2009;7 Suppl 5:S1–26 (quiz S27-28).
Poeck K, Hacke W. Neurologie. Springer-Verlag; 2006.
Boulton AJ. Lowering the risk of neuropathy, foot ulcers and amputations. Diabet Med. 1998;15(Suppl 4):S57-59.
Vinik A, Casellini C, Nevoret ML. Diabetic neuropathies. In: Feingold KR, Anawalt B, Boyce A et al (eds) Endotext. South Dartmouth; 2000.
Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9:1239–54.
Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335–7.
Theurich S, Malcher J, Wennhold K, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013;31:e59-63.
Kao JC, Liao B, Markovic SN, et al. Neurological complications associated with anti-programmed death 1 (PD-1) antibodies. JAMA Neurol. 2017;74:1216–22.
Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav. 2017;7:e00558.
Franques J, Chiche L, De Paula AM, et al. Characteristics of patients with vitamin B12-responsive neuropathy: a case series with systematic repeated electrophysiological assessment. Neurol Res. 2019;41:569–76.
Nardin RA, Amick AN, Raynor EM. Vitamin B(12) and methylmalonic acid levels in patients presenting with polyneuropathy. Muscle Nerve. 2007;36:532–5.
Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388:717–27.
Balke M, Wunderlicg G, Brunn A, et al. Chronic inflammatory demyelinating polyneuropathy. Fortschr Neurol Psychiatr. 2016;84:756–69.
Kandula T, Park SB, Cohn RJ, et al. Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev. 2016;50:118–28.
Moore RJ, Groninger H. Chemotherapy-induced peripheral neuropathy in pediatric cancer patients. Cureus. 2013;5(6):e124. https://doi.org/10.7759/cureus.124.
Bjornard KL, Gilchrist LS, Inaba H, et al. Peripheral neuropathy in children and adolescents treated for cancer. Lancet Child Adolesc Health. 2018;2:744–54.
Lieber S, Blankenburg M, Apel K, et al. Small-fiber neuropathy and pain sensitization in survivors of pediatric acute lymphoblastic leukemia. Eur J Paediatr Neurol. 2018;22:457–69.
Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118:828–38.
Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94:1451–7.
Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14:184–9.
Lehtinen SS, Huuskonen UE, Harila-Saari AH, et al. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2002;94:2466–73.
Varedi M, McKenna R, Lamberg EM. Balance in children with acute lymphoblastic leukemia. Pediatr Int. 2017;59:293–302.
Tay CG, Lee VWM, Ong LC, et al. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2017;64:e26471.
Kesting SV, Gotte M, Seidel CC, et al. One in four questioned children faces problems regarding reintegration into physical education at school after treatment for pediatric cancer. Pediatr Blood Cancer. 2016;63:737–9.
Boman KK, Hornquist L, De Graaff L, et al. Disability, body image and sports/physical activity in adult survivors of childhood CNS tumors: population-based outcomes from a cohort study. J Neurooncol. 2013;112:99–106.
Rueegg CS, Michel G, Wengenroth L, et al. Physical performance limitations in adolescent and adult survivors of childhood cancer and their siblings. PLoS ONE. 2012;7:e47944.
Verhagen E, van der Beek A, Twisk J, et al. The effect of a proprioceptive balance board training program for the prevention of ankle sprains: a prospective controlled trial. Am J Sports Med. 2004;32:1385–93.
Gollhofer A, Granacher U, Taube W, Melnyk M, Gruber M. motor control and injury prevention. Deutsche Zeitschrift für Sportmedizin. 2006;57:266–70.
Zhong D, Xiao Q, He M, et al. Tai Chi for improving balance and reducing falls: a protocol of systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e15225.
Goldhaber-Fiebert JD, Goldhaber-Fiebert SN, Tristan ML, Nathan DM. Randomized controlled community-based nutrition and exercise intervention improves glycemia and cardiovascular risk factors in type 2 diabetic patients in rural Costa Rica. Diabetes Care. 2003;26:24–9.
Granacher U, Mühlbauer T, Taube W, Gollhofer A, Gruber M. Sensorimotor training. In: Cardinale M, Newton R, Nosaka K, editors. Strength and conditioning: biological principles and practical applications. San Francisco: Wiley; 2011. p. 399–409.
Bruhn S, Kullmann N, Gollhofer A. The effects of a sensorimotor training and a strength training on postural stabilisation, maximum isometric contraction and jump performance. Int J Sports Med. 2004;25:56–60.
Taube W, Gruber M, Gollhofer A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol (Oxf). 2008;193:101–16.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12.
Dixit S, Maiya A, Shastry BA, Guddattu V. Analysis of postural control during quiet standing in a population with diabetic peripheral neuropathy undergoing moderate intensity aerobic exercise training: a single blind, randomized controlled trial. Am J Phys Med Rehabil. 2016;95:516–24.
Grewal GS, Schwenk M, Lee-Eng J, et al. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: a randomized controlled trial. Gerontology. 2015;61:567–74.
Streckmann F, Lehmann HC, Balke M, et al. Sensorimotor training and whole-body vibration training have the potential to reduce motor and sensory symptoms of chemotherapy-induced peripheral neuropathy-a randomized controlled pilot trial. Support Care Cancer. 2019;27:2471–8.
Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26:615–24.
Dhawan S, Andrews R, Kumar L, et al. A randomized controlled trial to assess the effectiveness of muscle strengthening and balancing exercises on chemotherapy-induced peripheral neuropathic pain and quality of life among cancer patients. Cancer Nurs. 2020;43(4):269–80.
Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018;26:1019–28.
Bland KA, Kirkham AA, Bovard J, et al. Effect of exercise on taxane chemotherapy-induced peripheral neuropathy in women with breast cancer: a randomized controlled trial. Clin Breast Cancer. 2019;19:411–22.
Kruse RL, Lemaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90:1568–79.
Kordi Yoosefinejad A, Shadmehr A, Olyaei G, et al. Short-term effects of the whole-body vibration on the balance and muscle strength of type 2 diabetic patients with peripheral neuropathy: a quasi-randomized-controlled trial study. J Diabetes Metab Disord. 2015;14:45.
Eftekhar-Sadat B, Azizi R, Aliasgharzadeh A, et al. Effect of balance training with Biodex Stability System on balance in diabetic neuropathy. Ther Adv Endocrinol Metab. 2015;6:233–40.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111.
Deeks J, Higgins, J. Statistical algorithms in review manager 5. In: Statistical methods group of the Cochrane Collaboration. 2010; p. 1–11.
Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567:305–7.
Blakeley B, McShane DG, Gelman A, Robert C, Tackett Jl. Abandon statistical significance. Am Stat. 2019;73:235–45.
Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337–50.
Fisch GS, Cohen IL, Jenkins EC, Brown WT. Screening developmentally disabled male populations for fragile X: the effect of sample size. Am J Med Genet. 1988;30:655–63.
Kanchanasamut W, Pensri P. Effects of weight-bearing exercise on a mini-trampoline on foot mobility, plantar pressure and sensation of diabetic neuropathic feet; a preliminary study. Diabet Foot Ankle. 2017;8:1287239.
Zhang X, Zhang Y, Gao X, et al. Investigating the role of backward walking therapy in alleviating plantar pressure of patients with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2014;95:832–9.
Abdelbasset WK, Alrawaili SM, Nambi G, et al. Therapeutic effects of proprioceptive exercise on functional capacity, anxiety, and depression in patients with diabetic neuropathy: a 2-month prospective study. Clin Rheumatol. 2020;39(10):3091–7.
Ahmad I, Noohu MM, Verma S, et al. Effect of sensorimotor training on balance measures and proprioception among middle and older age adults with diabetic peripheral neuropathy. Gait Posture. 2019;74:114–20.
Win M, Fukai K, Nyunt HH, Linn KZ. Hand and foot exercises for diabetic peripheral neuropathy: a randomized controlled trial. Nurs Health Sci. 2019;22(2):416–26.
Nadi M, Bambaeichi E, Marandi SM. Comparison of the effect of two therapeutic exercises on the inflammatory and physiological conditions and complications of diabetic neuropathy in female patients. Diabetes Metab Syndr Obes. 2019;12:1493–501.
Hung ES, Chen SC, Chang FC, et al. Effects of interactive video game-based exercise on balance in diabetic patients with peripheral neuropathy: an open-level, crossover pilot study. Evid Based Complement Alternat Med. 2019;2019:4540709.
Rojhani-Shirazi Z, Barzintaj F, Salimifard MR. Comparison the effects of two types of therapeutic exercises Frenkele vs. Swiss ball on the clinical balance measures in patients with type II diabetic neuropathy. Diabetes Metab Syndr. 2017;11 Supp 1:S29–32.
Sartor CD, Watari R, Passaro AC, et al. Effects of a combined strengthening, stretching and functional training program versus usual-care on gait biomechanics and foot function for diabetic neuropathy: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:36.
Lee K, Lee S, Song C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J Exp Med. 2013;231:305–14.
Song CH, Petrofsky JS, Lee SW, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–11.
Allet L, Armand S, de Bie RA, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53:458–66.
Gholami F, Nazari H, Alimi M. Cycle Training improves vascular function and neuropathic symptoms in patients with type 2 diabetes and peripheral neuropathy: A randomized controlled trial. Exp Gerontol. 2020;131:110799.
Dixit S, Maiya A, Shastry BA. Effects of aerobic exercise on vibration perception threshold in type 2 diabetic peripheral neuropathy population using 3-sites method: single-blind randomized controlled trial. Altern Ther Health Med. 2019;25:36–41.
Stubbs EB Jr, Fisher MA, Miller CM, et al. Randomized controlled trial of physical exercise in diabetic veterans with length-dependent distal symmetric polyneuropathy. Front Neurosci. 2019;13:51.
Gholami F, Nikookheslat S, Salekzamani Y, et al. Effect of aerobic training on nerve conduction in men with type 2 diabetes and peripheral neuropathy: a randomized controlled trial. Neurophysiol Clin. 2018;48(4):195–202.
Dixit S, Maiya A, Shastry B. Effect of aerobic exercise on quality of life in population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, randomized controlled trial. Qual Life Res. 2014;23:1629–40.
Dixit S, Maiya AG, Shastry BA. Effect of aerobic exercise on peripheral nerve functions of population with diabetic peripheral neuropathy in type 2 diabetes: a single blind, parallel group randomized controlled trial. J Diabetes Complic. 2014;28(3):332–9.
Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complic. 2006;20:216–23.
Seyedizadeh SH, Cheragh-Birjandi S, Hamedi Nia MR. The effects of combined exercise training (Resistance-Aerobic) on serum kinesin and physical function in type 2 diabetes patients with diabetic peripheral neuropathy (Randomized Controlled Trials). J Diabetes Res. 2020;2020:6978128.
Melai T, Schaper NCTHIJ, et al. Strength training affects lower extremity gait kinematics, not kinetics, in people with diabetic polyneuropathy. J Appl Biomech. 2014;30:221–30.
Ghavami H, Radfar M, Soheily S, et al. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, result of a randomized clinical trial. Agriculture. 2018;30:165–70.
Streckmann FHCL, Balke M, Schenk A, Oberste M, Heller A, Schürhöster A, Elter T, Bloch W, Baumann FT. Sensorimotor- and whole-body vibration training have the potential to reduce motor- and sensory symptoms of Chemotherapy-induced peripheral neuropathy—a randomized controlled pilot trial. Support Care Cancer. 2019;27(7):2471–8.
Schwenk M, Grewal GS, Holloway D, et al. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology. 2016;62:553–63.
Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25:493–9.
Kneis S, Wehrle A, Muller J, et al. It’s never too late - balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: results of a randomized controlled trial. BMC Cancer. 2019;19:414.
Vollmers PL, Mundhenke C, Maass N, et al. Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy. J Cancer Res Clin Oncol. 2018;144:1785–92.
Maharaj SS, Yakasai AM. Does a rehabilitation program of aerobic and progressive resisted exercises influence HIV-induced distal neuropathic pain? Am J Phys Med Rehabil. 2018;97:364–9.
Tumusiime DK, Stewart A, Venter FWD, Musenge E. The effects of a physiotherapist-led exercise intervention on peripheral neuropathy among people living with HIV on antiretroviral therapy in Kigali, Rwanda. S Afr J Physiother. 2019;75:1328.
Quigley PA, Bulat T, Schulz B, et al. Exercise interventions, gait, and balance in older subjects with distal symmetric polyneuropathy: a three-group randomized clinical trial. Am J Phys Med Rehabil. 2014;93:1–12 (quiz 13-16).
Lindeman E, Leffers P, Spaans F, et al. Strength training in patients with myotonic dystrophy and hereditary motor and sensory neuropathy: a randomized clinical trial. Arch Phys Med Rehabil. 1995;76:612–20.
Yakasai AM, Maharaj SS, Kaka B, et al. Does exercise program of endurance and strength improve health-related quality of life in persons living with HIV-related distal symmetrical polyneuropathy? A randomized controlled trial. Qual Life Res. 2020;29:2383–93.
Steimann M, Kerschgens C, Barth J. Rehabilitation bei chemotherapieinduzierter polyneuropathie. Onkologe. 2011;17:940–7.
Lemaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88:1385–98.
Mueller MJ, Tuttle LJ, Lemaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–38.
Kohut ML, McCann DA, Russell DW, et al. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav Immun. 2006;20:201–9.
Cobianchi S, Arbat-Plana A, Lopez-Alvarez VM, Navarro X. Neuroprotective effects of exercise treatments after injury: the dual role of neurotrophic factors. Curr Neuropharmacol. 2017;15:495–518.
Park JS, Hoke A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS ONE. 2014;9:e90245.
Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–38.
Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10.
Tomlinson DR, Gardiner NJ. Glucose neurotoxicity. Nat Rev Neurosci. 2008;9:36–45.
Kikkawa Y, Kuwabara S, Misawa S, et al. The acute effects of glycemic control on nerve conduction in human diabetics. Clin Neurophysiol. 2005;116:270–4.
Gollhofer A. Proprioceptive training: considerations for strength and power production. In: Komi PV, editor. Strenght and power in sport. 2nd ed. Oxford: Blackwell Publishing; 2003. p. 331–42.
Dermanovic Dobrota V, Hrabac P, Skegro D, et al. The impact of neuropathic pain and other comorbidities on the quality of life in patients with diabetes. Health Qual Life Outcomes. 2014;12:171.
Malik RA, Aldinc E, Chan SP, et al. Perceptions of painful diabetic peripheral neuropathy in South-East Asia: results from patient and physician surveys. Adv Ther. 2017;34:1426–37.
Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf). 2008;192:127–35.
Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–79.
Colberg SR. Key points from the updated guidelines on exercise and diabetes. Front Endocrinol (Lausanne). 2017;8:33.
Taube W, Gruber M, Beck S, et al. Cortical and spinal adaptations induced by balance training: correlation between stance stability and corticospinal activation. Acta Physiol. 2007;189:347–58.
Ahn S, Song R. Effects of Tai Chi Exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012;18:1172–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this article.
Conflicts of interest/Competing interests
Fiona Streckmann, Maryam Balke, Guido Cavaletti, Alexandra Toscanelli, Wilhelm Bloch, Bernhard Décard, Helmar Lehmann, and Oliver Faude have no conflicts of interest with regard to the content of this article.
Data availability
Data are available in the Electronic Supplementary Material.
Author contributions
FS and OF conceived the idea, and designed and conducted this meta-analysis. MB, WB, and HCL assisted in the design of the study. FS, AT, and OF developed the search strategy. FS and AT conducted the literature search and the study quality assessment, and extracted study information and outcome data. OF performed the statistical analyses. FS wrote the first paper draft with the assistance of MB and OF. GC and BD assisted in neurological and neurophysiological matters. All authors revised the manuscript for important intellectual content and approved the final version of the article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Streckmann, F., Balke, M., Cavaletti, G. et al. Exercise and Neuropathy: Systematic Review with Meta-Analysis. Sports Med 52, 1043–1065 (2022). https://doi.org/10.1007/s40279-021-01596-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-021-01596-6