Abstract
Background
Hemodialysis (HD) patients often experience cognitive deficits and reduced mobility. While studies have shown promising results of physical and/or cognitive training in older adults, their effects in HD patients remain understudied.
Aims
This study aimed to evaluate the impact of a 12-week intradialytic training program combining cognitive training with physical exercise on attention domains and spontaneous gait speed (SGS) in HD patients.
Methods
Forty-four HD patients were randomly assigned to either intradialytic cognitive and physical exercise training (EXP group; n = 22) or a standard care control group (CON group; n = 22). The EXP group performed intradialytic cycling and tablet-based cognitive training three days per week for 12 weeks. The primary outcome of the study was performance on the computerized test battery ‘Test of Attentional Performance.’ Secondary study outcome was patient mobility assessed by the four-meter SGS. Outcomes were assessed pre- and post-intervention.
Results
Significant group x time interaction was observed in alertness (F(1,41) = 6.15, p = 0.017) and SGS (F(1,41) = 18.33, p < 0.001) in favor of the EXP group. Within-group analysis revealed a significant pre–post decline in the CON group in alertness test (−26.7 s; p = 0.04) and an improvement of SGS in EXP group (+ 0.07 s; p < 0.001).
Discussion
This original study demonstrated that a combined physical and cognitive intradialytic training intervention led to improvements in SGS and preservation in alertness compared to a deterioration in the CON group.
Conclusion
Findings suggest that the intervention may serve as an effective tool to prevent the physical and cognitive decline in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with chronic kidney disease (CKD) treated with hemodialysis (HD) experience a decline in physical function and mobility, including difficulties with walking, balance, and coordination [1, 2]. This may be related to the effects of the disease itself and the treatment process, which cause muscle weakness and physical fatigue [3]. In addition, this patient population is at increased risk for cognitive impairment with significant deficits in attention and executive function [4]. Furthermore, cognitive decline in the general elderly population can affect gait parameters and a gait speed under 1 m/s associates with elevated risk for mortality and other detrimental health-related outcomes [5, 6]. When various affected cognitive components in middle-aged and older adults were analyzed, attention was the cognitive component most strongly related to gait speed [7]. Given the clear relationship between physical and cognitive performance, the impact of novel interventions targeting these two modalities should be explored to improve outcomes in this vulnerable population.
A physical exercise intervention showed to be effective in improving physical performance in HD patients [8]. Given the fact that gait speed is correlated with cognitive impairment, improving cognitive abilities could also be beneficial for improving patient’s physical performance. Nowadays, cognitive training is studied to improve physical performance-related outcomes and to counteract cognitive decline [9,10,11]. Most studies typically included sedentary or frail older adults as participants, and in general they have shown significant positive effects on physical performance [12,13,14]. The full extent of the effects of cognitive training, either alone or in combination with other non-pharmacologic approaches in HD patients remains unknown.
On the other hand, while existing literature suggests a positive effect of physical exercise on cognitive function, there is a lack of high-quality randomized controlled trial data and inconsistencies in the available evidence. Generally, the number of studies examining the effects of non-pharmacological interventions on cognitive function is low in HD population and they mostly involved interventions in the form of physical exercise. A walking program in HD patients showed an improvement in self-reported cognitive function [15] and its maintenance over a six-month period [16]. In a pilot randomized controlled trial [17], an intradialytic cycling group significantly reduced cognitive impairment compared with the control group. In contrast, intradialytic resistance exercise [18] and a chair stand exercise program [19] did not show significant effects on cognitive function. As far as the combination of cognitive and physical training is considered, there are no published studies except for an interventional pilot study in HD patients using an experimental group undergoing a cognitive training intervention, a second experimental group undergoing intradialytic cycling and a control group [20]. After three months, both intervention groups maintained the measured cognitive domains, whereas the control group showed a decline [19].
Assuming that both physical exercise and cognitive training have promising effects on cognitive performance in HD patients, it would be worthwhile to combine them. Therefore, the objective of this study was to examine the effects of cognitive training in conjunction with intradialytic cycling on cognitive and physical performance of dialysis patients. Considering that HD patients have significant attention deficits and physical performance deficits with reduced mobility, we chose attention cognitive domain and spontaneous gait speed (SGS) as key outcome variables. We hypothesized that the combination of cognitive and physical exercise interventions will reduce attention deficits and enhance gait speed, or at minimum, prevent the decline of these performance measures in a representative sample of dialysis patients.
Methods
Study design
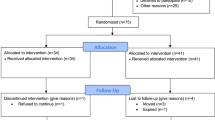
The present study was a single-blind randomized controlled trial aimed to investigate the effects of a bi-modal non-pharmacological intervention on cognitive parameters and SGS in HD patients. Patients were recruited from a local dialysis center in Ljubljana, Slovenia. The inclusion criteria were as follows: HD kidney replacement therapy (duration > 3 months), stable medical condition, absence of neurological diseases, and the ability to walk independently. The exclusion criteria were: active malignant or infectious disease, uncontrolled hypertension, angina pectoris (2–4 on the Canadian Cardiovascular Society scale), heart failure (3–4 on the New York Heart Association scale), severe cognitive impairment or dementia, history of limb amputation, or any other condition that could cause the patient to be clinically unstable. The study adhered to the ethical principles set forth in the Declaration of Helsinki of 1964 and received approval from the National Medical Ethics Committee (KME0120-474/2021/4). Prior to enrollment, all participants provided written informed consent. The clinical trial was registered with ClinicalTrials.gov (NCT05150444).
Participants
Out of the initial 72 individuals who were screened for eligibility, 44 were randomly assigned to either the intervention (EXP) or control (CON) group. Baseline characteristics of patients are presented in Table 1.
Values are expressed as mean ± SD, percent of subjects or index/score/grade. There were no statistically significant differences between the groups. Blood pressure was defined as the mean of the last three pre-dialysis blood pressure values. Phase angle measurements were performed with an 800μA current at a frequency of 50 kHz. n; number of subjects, BIA; bioimpedance performed using Body Composition Monitor, Fresenius AG, Bad Homburg, Germany; MoCA; Montreal Cognitive Assessment.
Study flow and protocol
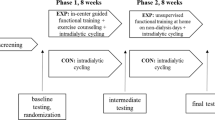
A detailed study protocol is described in Bogataj et al. (2022)[21]. After the screening process, all eligible patients who agreed to participate underwent baseline testing, that included tests of attentional performance and gait speed. They were then randomly assigned, using an online program (www.randomization.com), in a 1:1 ratio to either the EXP or CON group. Patients in the EXP group participated in a physical exercise and cognitive training program, while patients in the CON group received standard HD care. The intervention lasted for 12 weeks, with three training sessions per week (= 36 training sessions in total). Following the intervention, all patients were re-tested for the same outcomes as at baseline. The measurements were conducted on non-dialysis days, on the same day of the week and at the same time of day, with the same endpoint outcome assessors who were blinded to treatment allocation.
Physical exercise training
The EXP group performed an aerobic exercise session during dialysis. This type of training was delivered in the first two hours of dialysis, three days a week for 12 weeks, on a customized bed ergometer (BedBike, Lemco, Denmark). The cycling session began with a 5-min warm-up and ended with a 5-min cool-down. In the main part, we aimed to reach 30 min of exercising with a gradual increase in duration and resistance. The resistance was implied to each individual’s capability according to the rate of perceived exertion with maintaining the intensity level of 4th–5th grades on a 10-grade Borg scale, which was shown to be effective in this patient population [8, 22].
Cognitive training
Cognitive training was delivered on tablets through various games which target different cognitive subcategories. Each patient had their own profile on the CogniFit platform (CogniFit INC;USA), allowing the software to adjust the difficulty of the games based on individual performance to ensure that they were always being challenged without being overwhelmed. We selected the Personalized Brain Training package, which is designed to improve a wide range of cognitive skills, including memory, attention, perception, and coordination.
After the cycling session, the EXP group patients performed a 30–40-min cognitive training session. The length of the cognitive training session was based on a systematic review by Marusic et al. [11]. Research assistants supervised the sessions, helping with program initiation and ensuring the patients' understanding of the instructions for each game.
Outcome measures
Outcomes were assessed before and after the 12-week intervention. The primary outcome of the study was assessed by the computerized test battery ‘Test of Attentional Performance’ (TAP). This was based on its reported low learning effect [23], sensitivity to physical exercise [24], and the fact that attention is one of the most impaired cognitive abilities in HD patients [4]. From the TAP test battery [25], we selected the subtests Alertness, Selective attention and Divided attention. The tests were administered to each patient individually by a psychologist in a quiet, distraction-free room. The tests were relatively quick to administer and typically took 3–5 min each.
The Alertness subtest is designed to assess an individual's level of alertness and vigilance, which are important aspects of attentional processing. The test consists of a simple reaction time task in which the subject must react as quickly as possible to a stimulus [25]. The test assesses the speed and accuracy of the participant's response.
The Selective Attention subtest assesses the ability to pay selective attention to relevant information and to suppress unwanted reactions [25]. The test requires the subject to identify a target stimulus embedded in a series of distracting stimuli.
The Divided Attention subtest assesses a person's ability to attend to and process two different sources of information simultaneously [25]. The test requires the subject to perform two tasks simultaneously, one task being visual and the other auditory. In this "dual task," the subject must discriminate a visual stimulus (recognizing a square among crosses) and an auditory stimulus (recognizing irregularities in a sequence of sounds). The test measures the speed and accuracy of the subject's responses to both stimuli and assesses his or her ability to divide attention between the two tasks.
The secondary outcome of the study was the SGS, which is currently the most used test to assess mobility [26]. Patients were instructed to walk at their normal pace over a 6-m course, with a 4-m course marked in the middle for measurement. The average time of two attempts was calculated to evaluate the patients' gait speed (m/s).
Statistics
Statistical analysis was carried out using IBM SPSS version 29 (IBM Corporation, USA). GraphPad Prism software v9 was used for graph creation. Normality and sphericity were confirmed using Shapiro–Wilk and Mauchly's test. A repeated-measures ANOVA (2 × 2) with randomized group (EXP vs. CON) as between subject factor, and time (pre- and post-intervention) as a within subject factor was performed on TAP tests results and SGS. A paired-samples t-test was used to determine within-group differences over time. The study employed an intention-to-treat (ITT) analysis as the primary method of analysis. Cohen's d effect size (ES) was used to assess the observed differences magnitude for each group. A magnitude of 0.2 < ES ≤ 0.5 was considered small, while a magnitude of 0.5 < ES ≤ 0.8 and ES > 0.8 was treated as moderate and large [27].
The sample size calculation was performed using G*Power. It was calculated based on the results of a previous study [28] investigating the effect of aerobic exercise intervention on Alertness test results in multiple sclerosis patients. A paired t-test was conducted using the before and after results to calculate the required sample size. An alpha error probability of 0.05 and a 1-beta error probability of 0.80 were used, with an effect size of 0.565 taken from the abovementioned study. To account for an expected dropout rate of 20%, a sample size of 42 was calculated to ensure adequate statistical power to detect a difference between the two study groups.
Results
Tolerability, adverse events, and adherence
This bi-modal intervention in HD patients was well tolerated. No intervention-related adverse events were reported. One patient from the CON group was lost to follow-up due to transfer to another dialysis center because of colonization.
Adherence to the cycling and cognitive training programs was defined as the total number of completed sessions divided by the total number of all sessions. Adherence to cycling sessions was 79.9 ± 21.2%, with an average session duration of 37.6 ± 12.7 min. Cognitive training adherence was higher, reaching 84.2 ± 14.9% with an average session duration of 34 ± 4 min.
Reasons for skipping cycling sessions included pain, fatigue, hematoma, upper respiratory tract infection, COVID-19 infection, hypertension or hypotension, and dyspnea. Cognitive training sessions were mostly missed due to fatigue or COVID-19 isolation.
Cognitive tests
Pre- and post-TAP scores are shown in Table 2.
Repeated− measures ANOVA revealed a significant main effect of group (EXP vs. CON) x time (pre- vs. post-) interaction for Alertness test results (F(1,41) = o6.15, p = 0.017, ŋ2 = 0.13) in favor of the EXP group. Conversely, no significant group x time interaction was observed for Selective Attention (F(1,41) = 2.31, p = 0.136, ŋ2 = 0.053) and Divided Attention (F(1,41) = 0.001, p = 0.977, ŋ2 = 0.00).
Within-group analysis showed a significant pre–post difference only for the CON group for the Alertness test score, indicating a worsening in this cognitive domain (Table 2).
Values are expressed as mean ± sd: Significant within-group changes are signed with bold. ms: milliseconds; EXP: intervention group; CON: control group; ES: Cohen’s d effect size; CI: confidence interval; p: level of significance.
Spontaneous gait speed
Data analysis showed a statistically significant main effect for time x group interaction (F(1,41) = 18.33, p < 0.001, ŋ2 = 0.31). Within-group changes are graphically presented in Fig. 1. The baseline results for the SGS were 1.21 ± 0.19 m/s for the CON group and 1.20 ± 0.15 m/s for the EXP group. After the 12-week intervention, the EXP group increased its pace to 1.27 ± 0.14 m/s (p < 0.001, ES = −1.16), while the CON group decreased it to 1.2 ± 0.2 m/s (p = 0.155, ES = 0.32).
Discussion
This study investigated the effects of 12 weeks of combined physical and cognitive training on attention domains and SGS. Results from RM ANOVA showed a significant between-group interaction over time in alertness and SGS in favor to the EXP group. Within-group analysis in attention domains revealed a significant pre–post difference solely in the CON group in alertness test score, indicating a noticeable decline in this specific cognitive domain over the course of the study. Furthermore, the intervention resulted in a significant improvement in SGS compared with standard care.
While significant within-group improvements in attention domains were not observed, our intervention was found to be associated with the preservation of these domains. In contrast, the CON group exhibited a slight decline in selective attention and a significant decline in alertness. These results indicate that our intervention had a beneficial effect in maintaining attention abilities, providing support for its potential effectiveness in preventing cognitive decline in these domains. Our findings are consistent with gerontology studies, in which cognitive training and/or physical exercise training improved or preserved some, although not all, cognitive domains [29,30,31]. In a study with older adults, cognitive training showed a potential positive effect on executive function and other cognitive domains [30]. In a 6-month exercise intervention, older adults who did not exercise showed a decline in cognitive function, whereas those who participated in the exercise program maintained cognitive function over an 18-month period [31]. This study expanded previous findings on age-related cognitive decline to decline associated with end-stage renal disease, HD replacement therapy, and different age groups.
In a recent study conducted by Guo et al. (2022), attention was identified as the cognitive domain exhibiting the highest proportion of impairment among HD patients. The current study supports this finding by showing a significant reduction in alertness in the control group over a short period of time, indicating a rapid decline in this subdomain of attention. Importantly, Guo et al. (2022) also found a significant correlation between attention impairment and higher mortality rates in this population [32]. These findings highlight the potential impact of attention deficits on disease progression and prognosis, and serve as a starting point for future research investigating strategies to improve and maintain attention skills.
This is the first study examining the effects of a non-pharmacological intervention on the domains of attention in HD patients. Only one study investigated the effects of either intradialytic cycling or intradialytic tablet-based cognitive training on cognitive performance in HD patients [20]. Both intervention groups demonstrated the preservation of psychomotor speed and executive functions, in contrast to the control group, which experienced a decline. Our study aimed to exploit a synergistic effect of both interventions and to test the effects on the cognitive domain of attention using tests with high sensitivity and specificity. Our findings are consistent with the results of a study by Briken et al. [28], in which 8–10 weeks of aerobic exercise training in patients with progressive multiple sclerosis resulted in a significant improvement in alertness in the exercise group compared to the waitlist controls (p < 0.001).
A plethora of literature now exists linking cognitive processes, particularly executive functions and attention, with gait [33]. Improving SGS in the EXP group could potentially strengthen the evidence for a common neural substrate between these processes. The second noteworthy discovery pertains to the observed increase in gait speed by 0.07 m/s in the EXP group, which is indicative of a modest, yet meaningful and clinically significant improvement [34]. Improved SGS could have an impact on survival as gait speed has been identified as a subclinical indicator of physiological reserve and a marker of resilience to stress [35]. Previous research has shown that gait speed serves as a predictive indicator of all-cause mortality and cardiovascular events in HD patients [36].
Tests such as the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) are primarily used for screening mild cognitive impairment and dementia and may not be sufficiently sensitive to detect intervention effects [37, 38]. Additionally, these tests are prone to learning effects. Our study sought to address these limitations by utilizing specific and sensitive tests that minimize learning effects and provide more accurate assessments of cognitive function [25]. We conducted the baseline and post-test of our patients on non-dialysis days, thereby circumventing the potential confounding factors of pre- or post-dialysis fatigue effects. The patients demonstrated a high level of adherence throughout the entire study, presumably because the intervention was delivered during dialysis. Also, the intervention involved a multidisciplinary team of kinesiologists, physicians, nurses, and psychologists, which made the process more holistic and allowed for comprehensive patient care.
A limitation is that the results would benefit from replication in larger samples to improve their generalizability. Furthermore, it should be noted that the mean age of our sample was relatively low. Consequently, it is possible that older patients may exhibit lower tolerability to physical exercise interventions. Future research should include three separate groups, one participating solely in intradialytic cycling, cognitive training and the third group with both interventions. Due to the nature of the intervention, blinding of investigators and subjects was not possible. However, outcome assessors were blinded to group allocation. Consideration of these limitations helps to contextualize the results and highlight opportunities for future research to address these concerns and expand our understanding of cognitive function and mobility in this population.
Overall, incorporating both physical exercise and cognitive training into the care of HD patients may offer a comprehensive approach to improving their overall functioning. Further investigation is required to elucidate the underlying mechanisms and identify specific targets for preventive and therapeutic interventions. Additionally, there is a pressing need to develop tailored clinical care practices to help HD patients who experience cognitive and mobility impairments. Future research endeavors should also prioritize identifying risk factors associated with functional decline. By addressing these research gaps, we can advance our understanding and improve the management of cognitive and mobility impairments in this population.
Conclusion
In summary, our original study demonstrated that a combined physical and cognitive intradialytic training intervention in comparison to the standard care led to improvements in gait speed and maintenance in alertness compared to deterioration in the control group. Additionally, the intervention used was well tolerated, confirming the feasibility and safety of the training protocol. These findings suggest that the intervention may serve as an effective tool to prevent physical and cognitive decline in this patient population.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bučar Pajek M, Leskošek B, Vivoda T et al (2016) Integrative examination of motor abilities in dialysis patients and selection of tests for a standardized physical function assessment. Ther Apher Dial 20:286–294. https://doi.org/10.1111/1744-9987.12439
Bučar Pajek M, Pajek J (2018) Characterization of deficits across the spectrum of motor abilities in dialysis patients and the impact of sarcopenic overweight and obesity. Clin Nutr 37:870–877. https://doi.org/10.1016/j.clnu.2017.03.008
Zyga S, Alikari V, Sachlas A et al (2015) Assessment of fatigue in end stage renal disease patients undergoing hemodialysis: prevalence and associated factors. Med Arch 69:376. https://doi.org/10.5455/MEDARH.2015.69.376-380
Dixit A, Dhawan S, Raizada A et al (2013) Attention and information processing in end stage renal disease and effect of hemodialysis: a bedside study. Ren Fail 35:1246–1250. https://doi.org/10.3109/0886022X.2013.819768
Cesari M, Kritchevsky SB, Penninx BWHJ et al (2005) Prognostic value of usual gait speed in well-functioning older people-results from the health, aging and body composition study. J Am Geriatr Soc 53:1675–1680. https://doi.org/10.1111/J.1532-5415.2005.53501.X
Savica R, Wennberg AMV, Hagen C et al (2017) Comparison of gait parameters for predicting cognitive decline: the mayo clinic study of aging. J Alzheimers Dis 55:559–567. https://doi.org/10.3233/JAD-160697
Park H, Aul C, DeGutis J et al (2021) Evidence for a specific association between sustained attention and gait speed in middle-to-older-aged adults. Front Aging Neurosci 13:326
Bogataj Š, Pajek M, Pajek J et al (2020) Exercise-based interventions in hemodialysis patients: a systematic review with a meta-analysis of randomized controlled trials. J Clin Med 9:43. https://doi.org/10.3390/jcm9010043
Bogataj Š, Mesarič KK, Pajek M et al (2022) Physical exercise and cognitive training interventions to improve cognition in hemodialysis patients: a systematic review. Front Public Health 10:3813
Marusic U, Kavcic V, Giordani B et al (2015) Computerized spatial navigation training during 14 days of bed rest in healthy older adult men: effect on gait performance. Psychol Aging 30:334–340
Marusic U, Verghese J, Mahoney JR (2018) Cognitive-based interventions to improve mobility: a systematic review and meta-analysis. J Am Med Dir Assoc 19:484-491.e3. https://doi.org/10.1016/J.JAMDA.2018.02.002
Azadian E, Majlesi M, Jafarnezhadgero AA (2018) The effect of working memory intervention on the gait patterns of the elderly. J Bodyw Mov Ther 22:881–887. https://doi.org/10.1016/J.JBMT.2017.08.008
Ng TP, Feng L, Nyunt MSZ et al (2015) Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med 128:1225-1236.e1. https://doi.org/10.1016/J.AMJMED.2015.06.017
Smith-Ray RL, Makowski-Woidan B, Hughes SL (2014) A Randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact black older adults. Health Educ Behav 41:62S-69S. https://doi.org/10.1177/1090198114537068
Manfredini F, Mallamaci F, D’Arrigo G et al (2017) Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 28:1259–1268. https://doi.org/10.1681/ASN.2016030378
Baggetta R, D’Arrigo G, Torino C et al (2018) Effect of a home based, low intensity, physical exercise program in older adults dialysis patients: a secondary analysis of the EXCITE trial. BMC Geriatr 18:248. https://doi.org/10.1186/s12877-018-0938-5
Stringuetta Belik F, Silva Oliveira E, VR, Braga GP, et al (2018) Influence of intradialytic aerobic training in cerebral blood flow and cognitive function in patients with chronic kidney disease: a pilot randomized controlled trial. Nephron 140:9
Nakamura-Taira N, Horikawa N, Oka F et al (2021) Quasi-cluster randomized trial of a six-month low-intensity group-based resistance exercise for hemodialysis patients on depression and cognitive function: a 12-month follow-up. Health Psychol Behav Med 9:741–760. https://doi.org/10.1080/21642850.2021.1966302
Matsufuji S, Shoji T, Yano Y et al (2015) Effect of chair stand exercise on activity of daily living: a randomized controlled trial in hemodialysis patients. J Ren Nutr 25:17–24. https://doi.org/10.1053/J.JRN.2014.06.010
McAdams-DeMarco MA, Konel J, Warsame F et al (2018) Intradialytic cognitive and exercise training may preserve cognitive function. Kidney Int Rep 3:81–88. https://doi.org/10.1016/j.ekir.2017.08.006
Bogataj Š, Trajković N, Pajek M et al (2022) Effects of intradialytic cognitive and physical exercise training on cognitive and physical abilities in hemodialysis patients: study protocol for a randomized controlled trial. Front Psychol 13:62. https://doi.org/10.3389/FPSYG.2022.835486/BIBTEX
Bogataj Š, Pajek J, Buturović Ponikvar J et al (2020) Kinesiologist-guided functional exercise in addition to intradialytic cycling program in end-stage kidney disease patients: a randomised controlled trial. Sci Rep 10:5717
Zimmermann P, Fimm B (2017) Testbatterie zur Aufmerksamkeitsprüfung TAP. Version 2.3.1 (Test battery for attention testing TAP. Version 2.3.1). Herzogenrath, Psytest.
Noguera C, Sánchez-Horcajo R, Álvarez-Cazorla D et al (2019) Ten Years younger: practice of chronic aerobic exercise improves attention and spatial memory functions in ageing. Exp Gerontol 117:53–60. https://doi.org/10.1016/j.exger.2018.10.019
Zimmermann P, Fimm B (2021) Test of Attentional Performance 2.3.1 Available online: https://www.psytest.de/index.php?page=TAP-2-2&hl=en_US (accessed on 1 February 2021).
Peel NM, Kuys SS, Klein K (2013) Gait speed as a measure in geriatric assessment in clinical settings: a systematic review. J Gerontol: Ser A 68:39–46. https://doi.org/10.1093/GERONA/GLS174
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale
Briken S, Gold SM, Patra S et al (2014) Effects of exercise on fitness and cognition in progressive MS: a randomized. Controll Pilot Trial Mult Scler J 20:382–390. https://doi.org/10.1177/1352458513507358
Fu L, Kessels R, Maes J (2020) The effect of cognitive training in older adults: be aware of CRUNCH. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 27:949–962. https://doi.org/10.1080/13825585.2019.1708251
Ballesteros S, Prieto A, Mayas J et al (2014) Brain training with non-action video games enhances aspects of cognition in older adults: a randomized controlled trial. Front Aging Neurosci. https://doi.org/10.3389/FNAGI.2014.00277
Lautenschlager NT, Cox KL, Flicker L et al (2008) Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA - J Am Med Assoc 300:1027–1037. https://doi.org/10.1001/jama.300.9.1027
Guo Y, Tian R, Ye P et al (2022) Cognitive domain impairment and all-cause mortality in older patients undergoing hemodialysis. Front Endocrinol (Lausanne) 13:473. https://doi.org/10.3389/FENDO.2022.828162/BIBTEX
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23:329–342. https://doi.org/10.1002/MDS.21720
Perera S, Mody SH, Woodman RC et al (2006) Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54:743–749. https://doi.org/10.1111/J.1532-5415.2006.00701.X
Hardy SE, Perera S, Roumani YF et al (2007) Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 55:1727–1734. https://doi.org/10.1111/J.1532-5415.2007.01413.X
Lee YH, Kim JS, Jung SW et al (2020) Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol 21:1–11. https://doi.org/10.1186/S12882-020-01831-8/FIGURES/5
Dong Y, Sharma VK, Chan BPL et al (2010) The Montreal Cognitive Assessment (MoCA) is superior to the Mini-Mental State Examination (MMSE) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci 299:15–18. https://doi.org/10.1016/j.jns.2010.08.051
Sheehan B (2012) Assessment scales in dementia. Ther Adv Neurol Disord 5:349–358. https://doi.org/10.1177/1756285612455733
Funding
This study was funded by the Slovenian Research Agency (postdoctoral research project Z3-3212) and the ARRS research. and infrastructure program number P3-0323 (Renal diseases and renal replacement therapy).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Špela Bogataj, Katja Kurnik Mesarič and Aljaž Kren. Maja Pajek and Jernej Pajek supervised the study and acquired resources. The first draft of the manuscript was written by Špela Bogataj and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no relevant financial or non-financial interests to disclose.
Human and animal rights
This study was approved by the National Medical Ethics Committee of Slovenia (KME0120-474/2021/4).
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bogataj, Š., Pajek, M., Mesarič, K.K. et al. Twelve weeks of combined physical and cognitive intradialytic training preserves alertness and improves gait speed: a randomized controlled trial. Aging Clin Exp Res 35, 2119–2126 (2023). https://doi.org/10.1007/s40520-023-02511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02511-x