Abstract
Aims
To construct and validate an intraoperative hypothermia risk prediction model for elderly patients undergoing total hip arthroplasty (THA).
Methods
We collected data from 718 patients undergoing THA in a tertiary hospital from January 2021 to December 2022. Of these patients, 512 were assigned to the modeling group from January 2021 to April 2022, and 206 participants were assigned to the validation group from May 2022 to December 2022. A logistic regression analysis was performed to construct the model. The area under the curve (AUC) was used to test the model’s predictive ability.
Results
The incidence rate of intraoperative hypothermia was 51.67%. The risk factors entered into the risk prediction model were age, preoperative hemoglobin level, intraoperative blood loss, postoperative hemoglobin level, and postoperative systolic blood pressure. The model was constructed as follows: logit (P) = − 10.118 + 0.174 × age + 1.366 × 1 (preoperative hemoglobin level) + 0.555 × 1 (postoperative hemoglobin level) + 0.009 × 1 (intraoperative blood loss) + 0.066 × 1 (postoperative systolic blood pressure). Using the Hosmer–Lemeshow test, the P value was 0.676 (AUC, 0.867). The Youden index, sensitivity, and specificity were 0.602, 0.790, and 0.812, respectively. The incidence rates of intraoperative hypothermia in the modeling and validation groups were 53.15% and 48.06%, respectively. The correct practical application rate was 89.81%. This model had good application potential.
Conclusions
This risk prediction model has good predictive value and can accurately predict the occurrence of intraoperative hypothermia in patients who undergo THA, which provides reliable guidance for clinical work and has good clinical application value.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the global escalation of the aging process, the incidence of hip fractures in elderly patients has increased significantly [1]. Total hip arthroplasty (THA) is one of the most common surgical treatments for pain relief and physical function recovery. Although its perioperative course is relatively predictable, patients who undergo THA still experience various perioperative complications, especially intraoperative hypothermia (IOH) in geriatric patients.
IOH, defined as a core temperature (CT) < 36.0℃ during surgery, is a common complication among surgical patients [2]. In recent studies, the incidence rate of IOH has ranged from 26.6% to 65% in patients who undergo joint arthroplasty [3,4,5,6]. Elderly patients undergoing THA are at high risk of hypothermia during surgery, especially under general anesthesia (GA) [7]. GA and surgery affect the balance between heat production and loss. Previous studies have reported IOH rates of 13.2%–39% after THA [6, 8]. IOH is associated with several adverse effects, including blood loss, surgical site infection, disturbed drug metabolism, postoperative cardiovascular events, prolonged hospital stay, and mortality [9,10,11,12,13,14]. Despite these effects, IOH remains unresolved. Therefore, future studies should focus on IOH. In this era of personalized medicine, considering that elderly patients do not have the same risk factors for IOH, the development of risk prediction models for IOH is necessary.
A risk prediction model aims to predict the probability of future events, such as whether a patient will develop a disease or not [15]. A systematic review reported an overview of the current eight hypothermia risk prediction models for common surgeries, such as abdominal, thoracic, and gynecological surgeries [16]. In these studies, researchers identified an association between the occurrence of IOH and relevant predictors, such as age, weight, baseline temperature, and fluid volume [17,18,19]. However, several models failed to show pivotal data, such as sample size considerations, samples with missing data, and model validation, which may lead to a high risk of bias. Additionally, these predictors lead to existing prediction models for IOH that are unsuitable for elderly patients undergoing THA.
Therefore, to enrich IOH prevention for joint arthroplasty surgery, this study aimed to investigate the incidence of IOH, examine the risk factors, and construct and validate a risk prediction model for IOH in elderly patients undergoing THA. The risk prediction model was hypothesized to provide good discrimination and adequate calibration in clinical practice.
Methods
Study design and population
This was a prospective cohort study using a convenience sampling method to select patients who underwent THA in a tertiary general hospital with an annual volume of approximately 5000 orthopedic surgeries in Shanghai, China, from January 1, 2021, to December 31, 2022.
The inclusion criteria were as follows: patients (1) aged ≥ 60 years, (2) undergoing elective THA surgery, (3) with preoperative basal CT ranging from 36.5 to 37.5℃, (4) induced with GA, and (5) with good communication or without mental illness.
The exclusion criteria were as follows: patients (1) with premedication that affects the thermoregulatory center, (2) with central hyperthermia, (3) with abnormal thyroid function, (4) with infectious fever, (5) with body temperature regulation dysfunction, (5) who were unsuited for infrared tympanic thermometry, and (6) unwilling to participate in this study.
Sampling consideration and sample size
Based on the literature review and expert consultations, this study included 42 risk factors for data collection. These risk factors included the following: (1) patient’s general information (age, sex, height, weight, body mass index [BMI], past history [including surgery, anesthesia, smoking, and alcohol histories], comorbidities [including diabetes, hypertension, and cardiovascular and cerebrovascular diseases]); (2) preoperative physiological indicators (preoperative systolic blood pressure, preoperative diastolic blood pressure, preoperative hemoglobin level); (3) relevant surgical information (anesthesia classification, anesthesia time, operating time, awakening time from GA, fluid volume, flushing fluid volume, intraoperative blood transfusion, volume of blood transfusion, intraoperative blood loss, autologous blood transfusion, volume of urine, dosage of opioid drugs, dosage of muscle relaxants, postoperative systolic blood pressure, postoperative diastolic blood pressure, postoperative hemoglobin level); and (4) temperature indicators (preoperative CT, CT at induction of anesthesia, CT after 30 min of GA, CT after 60 min of GA, CT after 90 min of GA, CT after 120 min of GA, minimum intraoperative CT, maximum intraoperative CT, temperature in operating room, temperature of intraoperative fluids, temperature of intraoperative flushing fluids, temperature of intraoperative blood transfusion, CT after tracheal extubation, postoperative CT).
A preliminary experiment with 54 patients in this study showed that the incidence rate of IOH in elderly patients undergoing THA was 64.81% (35/54). The sample size was calculated based on the incidence of IOH in the preliminary experiment. According to the formula, sample size = (events per predictor variable × 5–10) × (1 + [15–20%])/0.6851, 373–778 samples could ensure the validity of the study results. A random seed within the SPSS version 25.0 software was used to draw 70% of the sample size as the modeling group and 30% as the validation group. Ultimately, 545 and 233 samples were included in the modeling and validation groups, respectively.
Study procedures
To provide reliable results, anesthesiologists and nurse anesthetists were trained in temperature management, temperature measurement, and data collection.
GA was induced with 0.02–0.05 mg/kg midazolam, 1.5–2.0 mg/kg propofol, 0.6 mg/kg rocuronium bromide, and 0.5–1 ug/kg remifentanil and maintained with 4.0–6.0 mg/kg propofol, 5–10 ug/kg remifentanil, 1.5–2 minimum alveolar concentration sevoflurane, and 0.1 mg/kg rocuronium bromide. The bispectral index (BIS) (Narcotrend® Narcotrend-Compact, MT monitorTechnik Gmbh& Co.KG, Germany) has been recommended to guide doses of anesthetics to achieve optimal anesthetic depth. The recommended range of BIS was 40–50 during anesthesia maintenance. Anesthesia induction, intraoperative anesthesia management, and awakening from GA were the primary responsibilities of anesthesiologists.
Operating room temperature was constant at 21–25℃, and room humidity ranged from 30 to 60%. All patients undergoing THA were covered with blankets, sheets, or surgical drapes perioperatively. Room-temperature intravenous fluids, room-temperature flushing fluids, and warm blood products were administered according to routine practice. Intraoperative temperature management was the responsibility of nurse anesthetists.
A noninvasive wireless ear thermometer (BodySTM® temperature transducer, Wismed, Beijing) was used to measure the CT of the participants. Before the induction of anesthesia, a wireless thermometer was inserted into the external auditory canal to measure the tympanic temperature. A previous study has reported that tympanic thermometers have good accuracy and precision for CT measurements [20]. The thermometer was paired with a monitoring module that continuously recorded the tympanic temperature, and the temperature data were uploaded to a terminal server for storage. Tympanic temperature readings were displayed on an electrocardiogram monitor (IntelliVue MX800® patient monitor, PHILIPS, Shanghai) and recorded on a data form by anesthesiologists every 10 min during the operation. The wireless ear thermometer was removed before the participants were discharged from the operating room to postoperative care unit. Intraoperative autologous transfusion (3000P® Blood Recycling machine; JINGJING, Beijing, China) was used to decrease the number of transfusions in patients with large intraoperative blood loss.
Data collection
This study included 718 elderly patients who underwent THA – 512 participants in the modeling cohort from January 2021 to April 2022 and 206 participants in the validation cohort from May 2022 to December 2022. A data form was developed to collect the demographic data and risk factors for IOH. The data form included the following contents: (1) demographic characteristics (age, sex, height, weight, BMI, past history [including surgery, history, smoking, and alcohol], comorbidities [including diabetes, hypertension, and cardiovascular and cerebrovascular diseases], systolic blood pressure, diastolic blood pressure, hemoglobin level); (2) surgical and anesthetic information (anesthesia classification, anesthesia time, operating time, awakening time from GA, fluid volume, flushing fluid volume, intraoperative blood transfusion, volume of blood transfusion, intraoperative blood loss, autologous blood transfusion, volume of urine, dosage of opioid drugs, dosage of muscle relaxants); and (3) temperature indicators (preoperative CT, CT at induction of anesthesia, CT after 30 min of GA, CT after 60 min of GA, CT after 90 min of GA, CT after 120 min of GA, minimum intraoperative CT, maximum intraoperative CT, temperature in operating room, temperature of intraoperative fluids, temperature of intraoperative flushing fluids, temperature of intraoperative blood transfusion, CT after tracheal extubation, postoperative CT).
The CT was measured before surgery. Nurse anesthetists monitored the CT every 10 min from the induction of anesthesia until the participants left the operating room. Continuous CT data during the operation were obtained using an electrocardiogram. CT < 36℃ was considered IOH. Subsequently, the training nurses obtained demographic data, relevant surgical information, anesthesia information, and physiological indicators from the HIS system of electronic medical records and recorded them in a data form.
Ethical consideration
This study was approved by the Ethics Committee (No. XHEC-D-2023–005). All patients voluntarily participated in this study and signed an informed consent form.
Statistical analyses
The SPSS version 25.0 software was used for the statistical analyses. Nomogram was drawn in R version 4.3.1 software to visualized. Regarding statistical description, measurement data following normal distribution are described as means ± standard deviations. Measurement data with non-normal distribution are described as medians and quartiles. Count data are presented as frequencies and percentages. T-tests were used to analyze normally distributed measurement data. The rank-sum test was used to measure data with a non-normal distribution. Chi-squared tests were used to analyze the count data. All risk factors were analyzed using single- and multi-factor logistic analyses. Logistic regression analysis was performed to construct a prediction model. The Hosmer–Lemeshow test and the area under the receiver operating characteristic (ROC) (AUC) were used to verify the prediction model. The practical application efficiency of the prediction model was verified by calculating the sensitivity, specificity, and accuracy of clinical data.
Results
Patient selection and flowchart of the study
Overall, 778 patients were screened for eligibility, of whom 33 and 27 patients in the modeling and validation groups, respectively, were excluded for the reasons explained in Fig. 1. In total, 718 valid patients were identified, with 512 and 206 in the modeling and validation groups, respectively.
Demographic characteristic
Among the 718 patients included in the analysis, IOH was observed in 371 patients, with an incidence rate of 51.67%. The 512 patients in the modeling group were divided into the hypothermia and non-hypothermia groups, with 272 patients in the hypothermia group, and the incidence rate of IOH was 53.15%. In the validation group, 99 patients had IOH, with an incidence rate of 48.06%.
In the univariate test, age, BMI, preoperative hemoglobin level, anesthesia time, volume of intraoperative fluids, volume of blood transfusion, intraoperative blood loss, postoperative systolic blood pressure, and postoperative hemoglobin level were significant factors that contributed to IOH (Table 1).
Prediction model of intraoperative hypothermia during total hip arthroplasty
Nine variables with statistical significance were identified as candidate predictors, and only five variables were retained in the model. The results showed that age, preoperative hemoglobin level, postoperative hemoglobin level, intraoperative blood loss, and postoperative systolic blood pressure were associated with IOH (Table 2). In the multivariate model, elderly patients (odds ratio [OR], 1.190; 95% confidence interval [CI], 1.140–1.242; P < 0.001) were at a high IOH risk. Patients with lower preoperative (OR, 1.255; 95% CI 1.178–1.365; P < 0.001) and postoperative hemoglobin (OR, 1.742; 95% CI 1.225–2.479; P = 0.002) levels were more likely to develop IOH. Intraoperative blood loss was associated (OR, 1.009; 95% CI 1.006–1.01; P < 0.001) with IOH. Postoperative systolic blood pressure was 1.068 (95% CI 1.024–1.115; P = 0.002) higher than the odds of IOH. The model was constructed as follows: logit (P) = − 10.118 + 0.174 × age + 1.366 × 1 (preoperative hemoglobin level) + 0.555 × 1 (postoperative hemoglobin level) + 0.009 × 1 (intraoperative blood loss) + 0.066 × 1 (postoperative systolic blood pressure).
Prediction effect of intraoperative hypothermia risk prediction model after internal verification during total hip arthroplasty
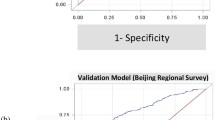
Using the Hosmer–Lemeshow test on the prediction model, we obtained a P value of 0.676, which indicates excellent calibration performance. ROC curves were used to test the sensitivity and specificity of the risk model for predicting IOH. The Youden index is a frequently used summary measure of ROC curves. The maximum Youden index is the best critical value of the prediction model. The AUC, Youden index, sensitivity, and specificity were 0.867, 0.602, 0.79, and 0.812, respectively (Fig. 2). The nomogram is shown in Fig. 3.
External validation of intraoperative hypothermia risk prediction model during total hip arthroplasty
The external validation of the prediction model was conducted on 206 patients. Ninety-nine patients underwent IOH, with an incidence rate of 48.06%. Of the 102 patients identified by the risk prediction model, hypothermia occurred in 89 patients, with a sensitivity of 89.90%. In total, 107 patients were predicted by the model; 96 patients were successfully predicted, with a specificity of 89.72%. The total accuracy of the model was 89.91% (89 + 96/206) (Table 3).
Discussion
IOH is the most common complication of surgery and can increase the risk of unfavorable perioperative events. Recently, most medical staff have recognized the several adverse effects of IOH and have gradually started to use risk prediction models and take early interventions to prevent IOH. Intraoperative prediction models for patients undergoing GA surgery can effectively predict and prevent IOH [21,22,23]. For example, the IOH risk prediction model developed by Dai et al. demonstrated helpful discrimination and adequate calibration [21]. THA has the characteristics of a large incision and trauma, but uses neuraxial or regional anesthesia, which is different from laparoscopic minimally invasive surgery and GA in patients with IOH. Neuraxial anesthesia impairs central thermoregulatory, reducing vasoconstriction and shivering threshold by 0.5℃ and elevating the sweating threshold by 0.3℃. This combined effect triples the interthreshold range, triggering a physiological response to the cold [24]. In addition, neuraxial anesthesia blocks the efferent nerves that regulate autonomic thermoregulatory defenses and dramatically impairs vasoconstriction and shivering [25]. Shortly after the administration of the neuraxial block, vasodilation shifts warm blood from the core to cooler peripheral tissues, resulting in a decrease in CT and redistribution hypothermia. Because of impaired thermoregulatory control, this drop in temperature may be sustained during anesthesia. In the case of regional anesthesia, redistribution of body heat is caused by centrally mediated vasodilation and direct inhibition of vasoconstriction (and shivering) in the regionally blocked area. Internal core-to-peripheral redistribution of body heat is the most important cause of hypothermia after neuraxial or regional anesthesia. To avoid bias, neuraxial and regional anesthesia were excluded from this study.
Based on a literature review, most joint replacement studies mixed THA and total knee arthroplasty (TKA) to construct risk prediction, which might affect the discrimination of risk factors and predictive values [26, 27]. Moreover, the incidence rate of IOH after TKA was significantly lower than after THA [8]. Therefore, it is necessary to study the risk factors and develop a predictive model for IOH in patients who undergo THA [28].
In this study, we observed an overall incidence rate of IOH of 51.67% in the patients who underwent THA. Older patients with a lower preoperative hemoglobin level before anesthesia, higher postoperative systolic blood pressure, lower postoperative hemoglobin level after surgery, and experienced more blood loss were susceptible to IOH. We proposed a risk prediction model that aimed to identify patients who underwent THA with a high risk of IOH. The Hosmer–Lemeshow test showed a P value of 0.676 (AUC, 0.867 [95% CI 0.836–0.898]), and the prediction success rate reached 89.81% in actual detection, confirming that this model has a good discriminative ability for prediction. Therefore, this model could be used to predict high risk factors for IOH in elderly patients who underwent THA and provide a reference for preventive interventions.
The mean age of patients in the IOH group was 75.83 ± 5.560 years, which had a significantly higher age than the non-hypothermia group (P < 0.001). Patients who undergo GA have an increased risk of IOH with advanced age [29,30,31]. The association between age and IOH may be due to a decrease in the thermoregulatory vasoconstriction threshold in patients under general anesthesia with age [18, 32]. Geriatric patients have a low metabolic rate, poor thermoregulatory ability, and high susceptibility to IOH.
IOH was more likely to occur at lower BMI (P = 0.001). In a previous study, being overweight was protective owing to the body fat maintaining heat balance by triggering vasoconstriction early when the CT decreased in obese patients [33]. Another study reported a strong positive association between serum leptin levels and fat [34]. Leptin stimulates energy consumption in brown adipose tissue and increases metabolic rate and body temperature. Therefore, the lower the BMI, the less leptin is secreted and the higher the likelihood of hypothermia.
A significant finding of this study was that univariate analysis showed that anesthesia time, volume of intraoperative fluids, and volume of blood transfusion were important factors influencing IOH. These results are consistent with those of a previous study, which reported that a longer duration of anesthesia (> 2 h) increased the risk of hypothermia [35]. In the present study, the administration of a lower volume of intraoperative fluid was significantly associated with IOH. However, this finding was inconsistent with those of previous studies [36, 37]. These variations could be due to differences in the technique of measurement and may be due to difference in sample population. From these results, clearly, IOH is associated with the volume of blood transfused during THA. Schmied et al. also found that intraoperative hypothermia increased blood transfusion requirements in patients undergoing THA [38, 39].
Compared with preoperative hemoglobin level before surgery, the mean hemoglobin level in the hypothermia group was significantly lower than that in the non-hypothermia group (P < 0.001), similar to the postoperative hemoglobin levels (P = 0.002). We found that greater intraoperative blood loss was an important factor in IOH development. A reasonable explanation is that with increasing intraoperative blood loss, hemoglobin level also decreases. IOH also reduces the adhesion ability of platelets and subsequent coagulation processes [40]. In this study, IOH was independently associated with lower hemoglobin levels and blood loss after THA. A previous study reported that maintaining body temperature during spinal surgery under GA could reduce blood loss and transfusion rates. However, the relationship among IOH, hemoglobin level, and blood loss in patients undergoing orthopedic surgery remains controversial [29, 41]. Considering that this study was limited to a single-center design, the relationship among IOH, hemoglobin levels, and blood loss requires further studies.
This study indicated that postoperative systolic blood pressure was an independent risk factor for IOH, which has not been previously reported [30, 42]. As the temperature decreases, blood vessels constrict, leading to an increase in blood pressure [43]. The effect of lower temperatures indicated a strong and acute effect of temperature on systolic blood. Decreases in temperature activate the sympathetic nervous system, leading to an increase in heart rate and the consequent risk of systolic blood pressure. A systematic review and meta-analysis found an inverse association between temperature and systolic blood pressure [44]. Patients with cardiovascular diseases may be more susceptible to temperature changes. Thus, improving IOH is required to manage and prevent cold-induced increases in systolic blood pressure. To date, the mechanisms underlying the relationship between temperature and blood pressure are not fully understood, and further studies are required.
Limitations
Compared with similar studies, the following limitations should be highlighted in this study. First, all the risk factors affecting IOH were included in the literature review; therefore, additional risk factors must be explored. Second, the sample size was small, and it was just a single-center study; multicenter, large sample data are required to further validate the prediction model. Third, this study revealed that patients with high postoperative systolic blood pressure were more susceptible to IOH, which differs from the findings of previous studies and requires further verification. Therefore, more well-designed studies are required to strengthen the predictive performance of risk models.
Conclusion
The risk prediction model has good predictive value and can accurately predict the occurrence of IOH in patients who undergo THA, which provides reliable guidance for clinical work and has good clinical application value.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Neuman MD, Feng R, Carson JL et al (2021) Spinal anesthesia or general Anesthesia for hip surgery in older adults. N Engl J Med 385:2025–2035. https://doi.org/10.1056/NEJMoa2113514
Sessler DI, Pei L, Li K et al (2022) Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): a multicentre, parallel group, superiority trial. Lancet 399:1799–1808. https://doi.org/10.1016/S0140-6736(22)00560-8
Ukrani RD, Arif A, Sadruddin A et al (2021) Intraoperative hypothermia in patients undergoing total knee arthroplasty: a cross-sectional study from a developing country. BMC Musculoskelet Disord 22:504. https://doi.org/10.1186/s12891-021-04390-7
Jildeh TR, Okoroha KR, Marshall NE et al (2018) The effect of intraoperative hypothermia on shoulder arthroplasty. Orthopedics 41:e523–e528. https://doi.org/10.3928/01477447-20180511-02
Kay AB, Klavas DM, Hirase T et al (2020) Preoperative warming reduces intraoperative hypothermia in total joint arthroplasty patients. J Am Acad Orthop Surg 28:e255–e262. https://doi.org/10.5435/JAAOS-D-19-00041
Simpson JB, Thomas VS, Ismaily SK et al (2018) Hypothermia in total joint arthroplasty: a wake-up call. J Arthroplasty 33:1012–1018. https://doi.org/10.1016/j.arth.2017.10.057
Sari S, Aksoy SM, But A (2021) The incidence of inadvertent perioperative hypothermia in patients undergoing general anesthesia and an examination of risk factors. Int J Clin Pract 75:e14103. https://doi.org/10.1111/ijcp.14103
Williams M, El-Houdiri Y (2018) Inadvertent hypothermia in hip and knee total joint arthroplasty. J Orthop 15:151–158. https://doi.org/10.1016/j.jor.2018.01.035
Shah A, Palmer AJR, Klein AA (2020) Strategies to minimize intraoperative blood loss during major surgery. Br J Surg 107:e26–e38. https://doi.org/10.1002/bjs.11393
Öner Cengiz H, Uçar S, Yilmaz M (2021) The role of perioperative hypothermia in the development of surgical site infection: A systematic review. AORN J 113:265–275. https://doi.org/10.1002/aorn.13327
Koh W, Chakravarthy M, Simon E et al (2021) Perioperative temperature management: a survey of 6 Asia-Pacific countries. BMC Anesthesiol 21:205. https://doi.org/10.1186/s12871-021-01414-6
Yan L, Tan J, Chen H et al (2023) A nomogram for predicting unplanned intraoperative hypothermia in patients with colorectal cancer undergoing laparoscopic colorectal procedures. AORN J 117:e1–e12. https://doi.org/10.1002/aorn.13845
Chung JY, Ting PC, Wu CL et al (2021) An analysis of the risk factors for postoperative complications and their effects on hospital stay in Taiwanese geriatric surgical patients. Asian J Anesthesiol 59:58–68. https://doi.org/10.6859/aja.202106_59(2).0003
Gala T, Shahzad N, Edhi AI et al (2020) Perioperative hypothermia in colorectal surgeries: are we doing enough to prevent it? JPMA J Pak Med Assoc 70:304–307. https://doi.org/10.5455/JPMA.294692
Jiang W, Wang J, Shen X et al (2020) Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care 43:925–933. https://doi.org/10.2337/dc19-1897
Yan L, Yao L, Zhao Q et al (2021) Risk prediction models for inadvertent intraoperative hypothermia: a systematic review. J Perianesth Nurs 36:724–729. https://doi.org/10.1016/j.jopan.2021.02.011
Yi J, Zhan L, Lei Y et al (2017) Establishment and validation of a prediction equation to estimate risk of intraoperative hypothermia in patients receiving General Anesthesia. Sci Rep 7:13927. https://doi.org/10.1038/s41598-017-12997-x
Emmert A, Gries G, Wand S et al (2018) Association between perioperative hypothermia and patient outcomes after thoracic surgery: a single center retrospective analysis. Medicine 97:e0528. https://doi.org/10.1097/MD.0000000000010528
Kim EJ, Yoon H (2014) Preoperative factors affecting the intraoperative core body temperature in abdominal surgery under general anesthesia: an observational cohort. Clin Nurse Spec 28:268–276. https://doi.org/10.1097/NUR.0000000000000069
Kameda N (2022) Clinical accuracy of non-contact forehead infrared thermometer and infrared tympanic thermometer in postoperative adult patients: a comparative study. J Perioper Pract 32:142–148. https://doi.org/10.1177/17504589211022314
Dai Z, Zhang Y, Yi J et al (2022) Validation of a prediction model for intraoperative hypothermia in patients receiving general Anesthesia. Int J Clin Pract 2022:6806225. https://doi.org/10.1155/2022/6806225
Wallisch C, Zeiner S, Scholten P et al (2021) Development and internal validation of an algorithm to predict intraoperative risk of inadvertent hypothermia based on preoperative data. Sci Rep 11:22296. https://doi.org/10.1038/s41598-021-01743-z
Mendonça FT, Ferreira JDS, Guilardi VHF et al (2021) Prevalence of inadvertent perioperative hypothermia and associated factors: a cross-sectional study. Ther Hypothermia Temp Manag 11:208–215. https://doi.org/10.1089/ther.2020.0038
Kurz A (2008) Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol 22:627–644. https://doi.org/10.1016/j.bpa.2008.06.004
Sessler DI (2016) Perioperative thermoregulation and heat balance. Lancet 387:2655–2664. https://doi.org/10.1016/S0140-6736(15)00981-2
Li L, Lu Y, Yang LL et al (2023) Construction and validation of postoperative hypothermia prediction model for patients undergoing joint replacement surgery. J Clin Nurs 32:3831–3839. https://doi.org/10.1111/jocn.16503
Matos JR, McSwain JR, Wolf BJ et al (2018) Examination of intra-operative core temperature in joint arthroplasty: a single-institution prospective observational study. Int Orthop 42:2513–2519. https://doi.org/10.1007/s00264-018-3967-y
Aceto P, Antonelli Incalzi R, Bettelli G et al (2020) Perioperative management of elderly patients (PriME): recommendations from an Italian intersociety consensus. Aging Clin Exp Res 32:1647–1673. https://doi.org/10.1007/s40520-020-01624-x
Akers JL, Dupnick AC, Hillman EL et al (2019) Inadvertent perioperative hypothermia risks and postoperative complications: a retrospective study. AORN J 109:741–747. https://doi.org/10.1002/aorn.12696
Chen HY, Su LJ, Wu HZ et al (2021) Risk factors for inadvertent intraoperative hypothermia in patients undergoing laparoscopic surgery: a prospective cohort study. PLOS ONE 16:e0257816. https://doi.org/10.1371/journal.pone.0257816
Chataule SM, Hazarika A, Jain K et al (2022) Preoperative forced-air warming strategy: Is it effective in averting intraoperative hypothermia in elderly trauma surgical patients? Cureus 14:e29305. https://doi.org/10.7759/cureus.29305
Hu Z, Li W, Liang C et al (2023) Risk factors and prediction model for inadvertent intraoperative hypothermia in patients undergoing robotic surgery: a retrospective analysis. Sci Rep 13:3687. https://doi.org/10.1038/s41598-023-30819-1
Li Y, Liang H, Feng Y (2020) Prevalence and multivariable factors associated with inadvertent intraoperative hypothermia in video-assisted thoracoscopic surgery: a single-center retrospective study. BMC Anesthesiol 20:25. https://doi.org/10.1186/s12871-020-0953-x
Izquierdo AG, Crujeiras AB, Casanueva FF et al (2019) Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients 11:2704. https://doi.org/10.3390/nu11112704
Kongsayreepong S, Chaibundit C, Chadpaibool J et al (2003) Predictor of core hypothermia and the surgical intensive care unit. Anesth Analg 96:826–833. https://doi.org/10.1213/01.ANE.0000048822.27698.28
Esen O, Yilmaz G, Aydin N (2020) Perioperative hypothermia in pediatric patients operated in a tertiary care center: Incidence and correlates. Pak J Med Sci 36:793–798. https://doi.org/10.12669/pjms.36.4.456
Mekete G, Gebeyehu G, Jemal S et al (2022) Magnitude and associated factors of intra-operative hypothermia among pediatric patients undergoing elective surgery: a multi-center cross-sectional study. Ann Med Surg (Lond). https://doi.org/10.1016/j.amsu.2022.103338
Schmied H, Kurz A, Sessler DI et al (1996) Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 347:289–292. https://doi.org/10.1016/s0140-6736(96)90466-3
Pan P, Song K, Yao Y et al (2020) The impact of intraoperative hypothermia on blood loss and allogenic blood transfusion in total knee and hip arthroplasty: a retrospective study. BioMed Res Int 2020:1096743. https://doi.org/10.1155/2020/1096743
Wolberg AS, Meng ZH, Monroe DM 3rd et al (2004) A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma 56:1221–1228. https://doi.org/10.1097/01.ta.0000064328.97941.fc
Tedesco NS, Korpi FP, Pazdernik VK et al (2014) Relationship between hypothermia and blood loss in adult patients undergoing open lumbar spine surgery. J Am Osteopath Assoc 114:828–838. https://doi.org/10.7556/jaoa.2014.169
Yi J, Lei Y, Xu S et al (2017) Intraoperative hypothermia and its clinical outcomes in patients undergoing general anesthesia: national study in China. PLOS ONE. https://doi.org/10.1371/journal.pone.0177221
MacMahon S, Peto R, Cutler J et al (1990) Blood pressure, stroke, and coronary heart disease Part 1. Prolonged differences in blood pressure: Prospective observational studies corrected for the regression dilution bias. Lancet 335:765–774
Wang Q, Li C, Guo Y et al (2017) Environmental ambient temperature and blood pressure in adults: a systematic review and meta-analysis. Sci Total Environ 575:276–286. https://doi.org/10.1016/j.scitotenv.2016.10.019
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant no.82172159), the National Natural Science Foundation of China (grant no.82072462), the Interdisciplinary Projects of Shanghai Jiao Tong University (grant no.YG2021QN56), Innovation research team of high-level local universities in Shanghai—SHSMU (grant no.ZDCX20212800) and the research project of Shanghai Jiao Tong University (grant no.Jyh2201).
Author information
Authors and Affiliations
Contributions
BZ: Conceptualization, Methodology, Writing—review and editing, ZZ: Data curation, Formal analysis, Software, Writing—review and editing, WQ: Data curation, Methodology, Software, QL: Data curation, Methodology, Software, QZ: Data curation, Methodology, Software, LJ: Methodology, Supervision, Validation, CW: Methodology, Supervision, Validation, XW: Validation, Supervision, Writing—review.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts or interest.
Ethical approval
This study was approved by the Ethics Committee (No. XHEC-D-2023–005).
Human and animal rights
The portions of this study involving human participants and human data were conducted in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of Xin Hua Hospital affiliated to Shanghai Jiao Tong University School of Medicine.
Informed consent
The patients provided their written informed consent to participate in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
zhao, B., zhu, Z., Qi, W. et al. Construction and validation of a risk prediction model for intraoperative hypothermia in elderly patients undergoing total hip arthroplasty. Aging Clin Exp Res 35, 2127–2136 (2023). https://doi.org/10.1007/s40520-023-02500-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02500-0