Abstract
Background
Though dementia rates vary by racial or ethnic groups, it is unknown if these disparities remain among those aged 90 or older.
Aims
To test this hypothesis, we used baseline clinical evaluation of 541 ethnically and racially diverse individuals participating in the LifeAfter90 Study to assess how associations between core demographic characteristics and measures of physical and cognitive performance differ across the racial/ethnic groups.
Methods
Participants in this study were long-term non-demented members of Kaiser Permanente Northern California. They were clinically evaluated and diagnosed with normal or impaired cognition (mild cognitive impairment and dementia) through an in-person comprehensive clinical assessment consisting of a detailed medical history, physical and neurological examination, functional, and cognitive tests.
Results
The average age at enrollment was 93.0 ± 2.6 years, 62.4% female and 34.2% non-Hispanic White. At initial evaluation 301 participants had normal cognition and 165 had mild cognitive impairment (MCI) and despite screening, 69 participants were determined to have dementia. Age, education, 3MS, FAQ and CDR scores were significantly associated with cognitive impairment (normal versus MCI and dementia), but not gender. There was a significant univariate association between race/ethnicity and cognitive impairment (p < 0.02) being highest among Black (57.4%) and lowest among Asian (32.7%) individuals. After adjustment for age, gender, and education, however, prevalence of cognitive impairment was not influenced by race or ethnicity.
Conclusion
Our results confirm the ability to reliably assess clinical diagnosis in a diverse sample of very old individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the US, people aged 90 and over are the fastest growing part of the population [1]. In 2010, this group represented 4.7% of the older population (≥ 65 years) and is estimated to reach 10% by 2050, representing 2% of total US population [2].
The incidence of dementia in this age group remains controversial as studies of this age group are often limited to small study size, presence of comorbidities, and difficulties distinguishing between cognitive and non-cognitive contributors to loss of functional abilities making diagnosis of cognitive impairment challenging [3]. Despite significant variability in studies, the literature suggests that both the incidence [4] and prevalence [5] of dementing disorders continue to increase with age and are particularly high among people beyond 90 years of age. Yet, current understanding of cognitive impairment in this age group is limited to studies of predominantly non-Hispanic White (White) individuals [3, 5,6,7]. Studies of younger diverse populations suggest marked differences in rates of dementia by racial/ethnic groups, with higher rates among Black and lower rates among Asian individuals [8], including those over age 90 [9]. A significant gap, therefore, exists in the epidemiology of dementia particularly among underrepresented ethno-racial groups of oldest old. Understanding dementia and its risk factors among ethno-racially diverse populations is particularly important given that the proportion of non-White elderly individuals continues to increase and estimated to be about 30% of oldest-old population in the U.S. by 2050 [2].
The LifeAfter90 Study is an ongoing prospective cohort study aimed to investigate life-course determinants of dementia incidence, cognitive decline, neuropathologic changes, and brain imaging markers in an ethnically and racially diverse cohort of individuals aged 90 years and older.
This manuscript describes the baseline clinical diagnostic evaluation of the first 541 participants enrolled into the LifeAfter90 Study.
Methods
Study participants
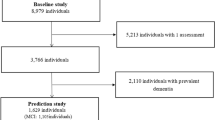
Participants in the LifeAfter90 Study are long-term members of Kaiser Permanente Northern California (KPNC) who were 90 years or older residing in the San Francisco Bay and Sacramento areas of California. Eligible individuals were KPNC members at some point between 1964 and 1992 and spoke English or Spanish. Exclusion criteria included inability to provide informed consent or a diagnosis of dementia or other neurodegenerative disease, hospice care, or dialysis in their electronic medical record at the time of enrollment. Study enrollment began in July 2018 and is ongoing. Figure 1 presents the flow chart of the participants’ enrollment as of March 2021. This analysis includes the first 541 individuals who were enrolled and completed the baseline clinical component with UC Davis from July 2018-February 2020.
The LifeAfter90 Study was approved by the KPNC, and UC Davis Institutional Review Boards and all enrolled participants provided informed consent.
Clinical evaluation components
UC Davis was responsible for the clinical component of this study which included detailed clinical evaluation of all participants approximately twice yearly. Six physicians trained by a behavioral neurologist (CD) with extensive experience in diagnosing cognitive impairment and dementia, conducted the clinical examination of the study participants. To facilitate participation of individuals with limited mobility, sensory impairment, or frailty, all evaluations are done in the participants’ homes. The evaluation consisted of collecting detailed medical history including history of cognitive complaints, a physical and neurological examination, cognitive function tests (Modified Mini-Mental State (3MS) examination [10] and Clinical Dementia Rating (CDR) [11]), assessment of physical performance (Short Physical Performance Battery(SPPB) [12]) and assessment of functional impairment (Functional Activities Questionnaire(FAQ) [13]), tools commonly used in the clinical assessment of presence and severity of cognitive impairment [14, 15] (Supplementary Table S1).
Clinical evaluation instruments
Modified mini-mental state exam (3MS)
The 3MS, a modified version of Mini-Mental State Exam, includes the following domains: verbal fluency, reasoning/judgment, expressive language, visual construction, immediate and delayed free verbal recall, and cued verbal recall [10]. Several studies documented its superiority to MMSE in discriminating different levels of cognitive impairment [16,17,18,19].
Clinical dementia rating (CDR)
The CDR was administered to the participant and the informant, if one was available at the time of the clinical evaluation. It collected information through semi-structured interviews to assess the presence and level of cognitive impairment in six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care [11].
Functional activities questionnaire (FAQ)
The FAQ is a 12-item scale that collected information on instrumental activities of daily living [13]. A modified version of FAQ was used in this study enabling distinction between different causes (cognitive vs other (sensory or physical)) for functional impairment. It was administered primarily to the participant and was supplemented with the information provided by the informant, when available. The modified FAQ allowed for the assignment of separate sub-scores indicating if functional impairment was due to cognitive or non-cognitive limitations including hearing or visual impairment, changes in upper or lower extremities, or balance problems (Supplementary table S2).
Short physical performance battery (SPPB)
SPPB was used to assess balance, gait, and lower extremity strength in older individuals [12] known to be associated with cognitive decline [20]. The SPPB consists of 5 tests of physical ability: (1) side-by-side stand, (2) semi-tandem stand, (3) tandem stand, (4) timed walk (3 or 4 m), and 5) time to arise from a chair 5 times. Each physical activity is timed and scored by ability and time to complete, with a range of 0 indicating no ability to 12 indicating full ability at all tasks.
Clinical diagnosis and adjudication
Diagnosis of dementia was made using the criteria of Diagnostic and Statistical Manual of Mental Disorders Fourth Edition and required (1) impairment in memory and one additional cognitive domain; (2) decline from a previous level of functioning due to cognition; and (3) presence of cognitive deficits not only during delirium [21] as updated by National Institute on Aging, Alzheimer’s Association [15]. Diagnosis of mild cognitive impairment (MCI) was consistent with Petersen’s criteria [22] and required (1) subjective complaints; (2) impairment in one or more cognitive domains; (3) no functional impairment due to cognition; and (4) no dementia. Final clinical diagnosis of the participants was determined in the following sequence: (1) at the end of each clinical evaluation, the physicians documented their initial impression based on the overall information collected during the assessment that included history, physical and neurological examination along with “bedside” cognitive and functional testing as indicated by recent recommendations [15], and assigned the participant a diagnosis of either normal cognition (NC), mild cognitive impairment (MCI), or dementia; (2) a behavioral neurologist reviewed the documented results of the clinical evaluation and adjudicated the final clinical diagnosis. Discrepancies between the initial clinical impression and final clinical diagnosis were reviewed and discussed during quality control and reliability sessions conducted monthly until the final consensus diagnosis was reached.
Statistical analysis
The primary dependent variable was the clinical diagnosis of NC, MCI, or dementia. Due to the relatively low number of individuals with dementia in this subsample, however, clinical diagnosis was operationalized to normal or impaired cognition (MCI or dementia) in the final analysis. Independent variables of interest included demographic variables (race/ethnicity, gender, age, and education), self-described measures of general health (health perception, smoking status, medical history including presence of cardiovascular, cerebrovascular, or vascular diseases), physical (SPPB and FAQ non-cognitive scores) and cognitive performances (3MS, CDR, CDR sum of boxes (CDR SOB [23]) and FAQ cognitive scores). Race/ethnicity was captured in five categories: Asian, Black, Latino, White, and multiracial. Education was coded as three categories: achievement up to High School graduation or GED, any college education, and any graduate education. Missing values for all variables were checked for randomness using Little’s missing at random test [24] and were replaced with the mean of the variable if more than 1% of data was missing randomly for quantitative variables. Only the SPPB total score had more than 1% missing values (1.8%) and were replaced with the mean SPPB score of 6.29.
We first generated basic descriptive statistics for all participants. Differences between the participants across racial/ethnic groups as well as subgroups of cognition were compared using independent t-test and Pearson’s chi-square test.
Multiple logistic regressions were performed to define independent risk factors for cognitive impairment and control for potential confounders.
We used STATA 13.0 statistical software for all analysis.
Results
Demographic characteristics of study participants
Tables 1 and S3 summarize characteristics of the 541 participants included in this analysis by clinical diagnosis (Table 1), and by race and ethnicity (Table S3). The average age at enrollment was 93.0 ± 2.6 years [range 90–105], and most were female (62.4%). Educational achievement was generally high with most having either some college (40.6%) or graduate school (19.0%) experience. All demographic characteristics differed significantly across the racial/ethnic subgroups. Multiracial and Black individuals had the highest proportion of women (71.7% and 70.4%, respectively) while Asian individuals had the lowest (48.7%). White, Black, and Latino individuals had the same average age (93.2 years) at enrollment, but Asian and Multiracial individuals were significantly younger (92.3 and 92.7 years, respectively). Educational achievement varied significantly by race and ethnicity (Supplement Table 3). The median educational achievement for White, Black and Asian individuals was some level of college education, whereas the median level of educational achievements for Latino individuals was high-school or GED. Health perception and prevalence of vascular risk factors also varied by race/ethnicity with White individuals having the highest degree of perceived health and Black individuals having the greatest prevalence of vascular risk factors.
Cognition
Despite exclusion of a prior diagnosis of dementia in the medical record, almost 13% of the individuals evaluated received a clinical diagnosis of dementia at baseline and 31% were diagnosed with MCI.
Age, gender, and education in relation to cognition
Initial analysis included demographic characteristics (gender, age, and education). In univariate analyses, increasing age was associated with a higher likelihood of cognitive impairment (p < 0.001). College and graduate school achievement were more common among cognitively normal individuals (p = 0.023). Gender was not associated with cognitive impairment (p = 0.931). In a multiple logistic regression model using these demographic variables, age, college and graduate school education, but not gender, were associated with a higher likelihood of cognitive impairment versus cognitively normal.
Race/ethnicity and cognition
There was a significant univariate association between race/ethnicity and cognitive impairment (p < 0.02) being highest among Black (57.4%) and lowest among Asian (32.7%) individuals. After adjusting for age, gender, and education, however, there were no race/ethnic differences in the likelihood of cognitive impairment,
Vascular disease and cognition
Vascular risk factors (hypertension, hypercholesterolemia, or diabetes) as well as cardiovascular diseases (Table 1) were significantly more prevalent among participants with normal cognition, whereas history of any cerebrovascular disease was significantly more prevalent among the participants with dementia.
Cognitive performance
Association of CDR with diagnosis
As expected, there was a highly significant association between CDR ratings, either as CDR score or CDR sum of boxes (SOB), and clinical diagnosis for this group. Specifically, only 15.8% of individuals determined to be cognitively normal had a CDR score 0.5 as compared to 74.3% of participants with cognitive impairment (Table 1). The association between CDR SOB and diagnosis did not vary after adjusting for age, gender, race/ethnicity, or educational achievement.
Cognitive performance measures as predictors of cognitive status
After adjusting for age, gender, education, physical performance (SPPB) and function (non-cognitive FAQ), lower 3MS remained a significant predictor of impaired cognition across all race and ethnic groups. Similarly, higher cognitive FAQ scores remained a significant predictor of cognitive impairment for all subgroups except for Black and multiracial individuals (Table 2).
Discussion
The LifeAfter90 Study is an unprecedented epidemiologic study of ethnically diverse oldest-old individuals. The accurate diagnosis of cognitive impairment is critical to assessing the impact of early-life risk and protective factors on dementia incidence and possibly health disparities among diverse populations. Our results indicate that, not only can the diagnosis of cognitive impairment, using accepted clinical diagnostic guidelines [14, 15, 22], be made amongst a racially and ethnically diverse group of individuals 90 years of age and older, but that the utility of “beside” measures of cognitive ability [15], particularly the 3MS and the cognitive component of the FAQ are supported by the results of a detailed and comprehensive clinical evaluation performed in the home. Specifically, lower 3MS remained a significant predictor of impaired cognition across all racial and ethnic groups after adjusting for age, gender, education, and measures of physical performance, suggesting that 3MS and functional disability due to cognitive impairment measured by the FAQ have the potential for use as a screening tool in this population. Moreover, we found that the relationship between scores on these general measures of cognition, function, and clinical diagnosis do not vary by race or ethnicity supporting an unbiased approach to clinical assessment despite widely varying degrees of educational attainment and physical abilities.
Dementia at baseline
Although individuals were screened by review of their medical histories to exclude prevalent dementia, we expected to enroll some percentage of individuals with dementia. We believe the percentage of individuals with dementia at the baseline evaluation (13%) in this subsample was due partially to lack of regular cognitive surveillance, given the recognized exponential rise in yearly dementia incidence after age 90 [4]. It could also reflect the difficulty of diagnosing dementia among the oldest old in primary care [3].
Impact of sex differences
Unlike most prevalence studies suggesting higher prevalence in women than in men [5, 25,26,27], we did not find any significant differences in prevalence of cognitive impairment between men and women in this study. This might be explained by the small sample size and exclusion of the individuals with diagnosis of dementia in their medical record. Further analysis for cognitive impairment among men and women as well as measures of survival after diagnosis of cognitive impairment, will allow for further examination of gender differences in incident dementia among this diverse group of oldest old.
Vascular risk factor assessment
We found a significantly higher prevalence of vascular risk factors and cardiovascular disease amongst individuals with normal cognition. This result is similar to other studies showing that hypertension and high cholesterol levels have an inverse association with dementia in the oldest-old [28,29,30]. For this analysis, however, the presence of these diseases was based primarily on participants self-report. Those with normal cognition, therefore, may have been more likely to report these comorbidities compared to those with some cognitive impairment. Additionally, we did not collect information on the duration of, or treatment for, these diseases. Future studies using life-course data obtained from the Kaiser health system will focus on early-life health factors to further understand risk and resilience in this unique cohort. Conversely, a history of cerebrovascular disease (described as transient ischemic attack (TIA) or stroke) was significantly more common among those individuals with dementia consistent with vascular contributions to cognitive impairment as previously noted among participants in the 90 + study[31].
Strengths and limitations
One of the strengths of this study is the detailed in home assessment performed by the clinicians with clinical adjudication by a behavioral neurologist similar to that used in the East Boston Dementia study [32] and refined by experience in the 90 + study [3]. Importantly, the clinical adjudicator was blind to race and ethnicity. The clinical evaluations were completed in the homes of the participants enabling individuals of all levels of physical and cognitive abilities to participate thereby reducing common forms of selection bias.
The potential limitation of this study might be diagnostic misclassification. This could be due to several reasons, including the difficulty of determining whether functional limitations are due to cognitive impairment or physical limitations (sensory and motor deficits, comorbidities) or the combination of both [3, 12]. Moreover, diagnosis was obtained without assistance of brain imaging or detailed neuropsychological testing, both of which are tools of proven value in the differential diagnosis of dementia [15]. Generalizability of the findings might be another limitation of this study as participants have been long-term members of KPNC system with higher average educational achievement and available access to the health care as compared to the general population.
Conclusion
In conclusion, the oldest old are a select group of the growing population who may escape major illnesses or delay onset toward the end of life. Preliminary analysis of this population finds excellent consistency between “bedside”[15] cognitive and functional measures and the clinical diagnosis of cognitive impairment blindly adjudicated by a behavioral neurologist that were unaffected by the race or ethnicity of the participants. Lifestyle, health, and genetic factors that could be of great importance to understanding dementia disparities in this oldest-old population will be further explored once enrollment is complete and the clinical diagnostic data is linked with the decades of prospectively collected health information and detailed neuropsychological testing available for the participants of LifeAfter90 study.
Data availability
The funder had no role in study design, subject recruitment, data collection and analysis, and preparation of the manuscript.
References
He W, Muenchrath MN (2011) 90+ in the United States: 2006–2008. US Census Bureau. Am Comm Survey Rep 17:1–28
Vincent GK, Velkoff VA (2010) The next four decades, the older population in the United States: 2010 to 2050, current population reports. U.S. Census Bureau, Washington, DC, pp 25–1138
Brumback-Peltz C, Balasubramanian AB, Corrada MM et al (2011) Diagnosing dementia in the oldest-old. Maturitas 70:164–168
Corrada MM, Brookmeyer R, Paganini-Hill A et al (2010) Dementia incidence continues to increase with age in the oldest old the 90+ study. Ann Neurol 67:114–121
Corrada MM, Brookmeyer R, Berlau D et al (2008) Prevalence of dementia after age 90: results from the 90+ study. Neurology 71:337–343
Edland SD, Rocca WA, Petersen RC et al (2002) Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester. Minn Archives of Neurology 59:1589–1593
Ruitenberg A, Ott A, Swieten JCV et al (2001) Incidence of dementia : does gender make a difference ? Neurobiol Aging 22:575–580
Mayeda ER, Glymour MM, Quesenberry CP et al (2016) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12:216–224
Gilsanz P, Corrada MM, Kawas CH et al (2019) Incidence of dementia after age 90 in a multiracial cohort. Alzheimers Dement 15:497–505
Teng EL, Chui HC (1987) The modified mini-mental state (3MS) examination. J Clin Psychiatry 48:314–318
Morris JC (1993) The clinical dementia rating (cdr): Current version and scoring rules. Neurology 43:2412–2414
Guralnik JM, Simonsick EM, Ferrucci L et al (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49:85–94
Marshall GA, Zoller AS, Lorius N et al (2016) Functional activities questionnaire items that best discriminate and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res 12:493–502
Knopman DS, Dekosky ST, Cummings JL et al (1999) Practice parameter : diagnosis of dementia ( an evidence-based review ). Neurology. https://doi.org/10.1212/WNL.56.9.1143
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Bassuk SS, Murphy JM (2003) Characteristics of the modified mini-mental state exam among elderly persons. J Clin Epidemiol 56:622–628
Bland RC, Newman SC (2001) Mild dementia or cognitive impairment : the modified mini-mental state examination (3MS ) as a screen for dementia. Can J Psychiatry 46:506–510
Ingram R (2017) Assessment of cognitive impairment in older research participants. Biomed J Sci Techn Res 1:842–846
Kristjunsson B, Hill GB (1997) Community screening for dementia : the mini mental state exam ( MMSE ) and modified miniemental state exam (3MS ) compared. J Clin Epidemiol 50:377–383
Handing EP, Leng XI, Kritchevsky SB et al (2020) Association between physical performance and cognitive function in older adults across multiple studies: a pooled analysis study. Innov Aging 4:1–8
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. DC. American Psychiatric Association, Washington
Petersen RC, Stevens JC, Ganguli M et al (2001) Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review). Neurology 56:1133–1142
Lynch CA, Walsh C, Blanco A et al (2006) The clinical dementia rating sum of box score in mild dementia. Dement Geriatr Cogn Disord 21:40–43
Rubin DB (1976) Inference and missing data. Biometrika 63:581–592
Börjesson-Hanson AEE, Gislason T, Skoog I (2004) The prevalence of dementia in 95 year olds. Neurology 63:2436–2438
Ebly EMPI, Hogan DB, Fung TS (1994) Prevalence and types of dementia in the very old: results from the Canadian study of health and aging. Neurology 44:1593–1600
LA Heeren TJ, Hijmans W, Rooymans HG (1991) Prevalence of dementia in the ‘oldest old’ of a Dutch community. J Am Geriatr Soc 39:755–759
Corrada MM, Hayden KM, Paganini-Hill A et al (2017) Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ study. Alzheimers Dement 13:103–110
Li G, Rhew IC, Shofer JB et al (2007) Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc 55:1161–1167
Mielke MM, Zandi PP, Sjogren M et al (2005) High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology 64:1689–1695
Peltz CB, Corrada MM, Berlau DJ et al (2012) Cognitive impairment in nondemented oldest-old: Prevalence and relationship to cardiovascular risk factors. Alzheimers Dement 8:87–94
Evans DA, Funkenstein HH, Albert MS et al (1989) Prevalence of Alzheimer’s disease in a community population of older persons higher than previously reported. JAMA 262:2551–2556
Acknowledgements
We are grateful to all participants and staff for making this research possible.
Funding
Funding was provided by the National Institute on Aging, grant numbers: RF1 AG056519 (RAW), P30 AG072972 (CD). The NIH had no input as to the content of this article.
Author information
Authors and Affiliations
Contributions
KEV, SJ, ALL, AD, CG, OP, and RG were involved in study coordination, data collection and data quality assurance; DP, CDC, MMC, CHK, RAW, PG, KEV and DM were responsible for data management, analysis, interpretation of results and writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Human and animal participants
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. He study was approved by the Bioethics Committee of the University of California at Davis on 2/18/2018 under IRB approved protocol #1180809. All subjects signed informed consent prior to enrollment in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petrosyan, D., Corrada, M.M., Kawas, C.H. et al. Assessing cognitive impairment in an ethnically diverse cohort of oldest-old: the life after 90 study. Aging Clin Exp Res 35, 979–986 (2023). https://doi.org/10.1007/s40520-023-02368-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-023-02368-0