Abstract
Background
Acrylamide, a component of fried foods, has been associated with several negative health outcomes. However, the relationship between dietary acrylamide and osteoporotic fractures has been explored by a few cross-sectional studies.
Aims
To investigate if dietary acrylamide is associated with the onset of fractures in North American participants at high risk/having knee osteoarthritis (OA), over 8 years of follow-up.
Methods
A Cox’s regression analysis, adjusted for baseline confounders was run and the data were reported as hazard ratios (HRs) and 95% confidence intervals (CIs). Dietary acrylamide intake was assessed at the baseline using a food frequency questionnaire and categorized in tertiles (T), whilst fractures’ history was recorded using self-reported information.
Results
Altogether, 4,436 participants were included. Compared to participants with lower acrylamide intake (T1; < 3,313 μg), those with a higher acrylamide intake (T3; > 10,180 μg) reported a significantly higher risk of any fracture (HR = 1.37; 95% CI 1.12–1.68; p for trend = 0.009), forearm (HR = 1.73; 95% CI 1.09–2.77; p for trend = 0.04), spine (HR = 2.21; 95% CI 1.14–4.31; p for trend = 0.04), and hip fracture (HR = 4.09; 95% CI 1.29–12.96; p for trend = 0.046).
Conclusions
Our study is the first to report that high dietary acrylamide may be associated with an increased risk of osteoporotic fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acrylamide is a vinyl monomer principally derived from chemical industries to produce polymers for water treatment, oil drilling, paper making and mineral processing [1]. Acrylamide was firstly evaluated by the International Agency for Research on Cancer and identified as potentially carcinogenic to humans [2]. As a food component, acrylamide can be formed during the thermal processing of carbohydrate-rich foods, especially deep-frying, oven-baking, and roasting [3].
The amount of acrylamide in cooked foods is determined by cooking temperature and time and the quantity of reducing sugar and asparagine in raw foods [4]. The World Health Organization (WHO) attempted to evaluate the average exposure to acrylamide in foods, however, this is difficult to measure with traditional instruments available in nutritional research [5]. At the same time, increasing research is reporting that higher dietary acrylamide intake could be associated with a higher risk of cancer, in particular genito-urinary [6, 7] or negative outcomes in pregnancy and in newborns [8, 9].
Recently Pan et al. found that acrylamide exposure in animal cell lines led to oxidative stress as shown by a significant increase in reactive oxygen species (ROS) and malondialdehyde (MDA) levels and glutathione (GSH) reduction, all factors associated with osteoporosis and fractures in older people [10]. Inflammatory response was observed based on dose-dependent levels of dietary acrylamide in relation to pro-inflammatory cytokines tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) [10]. In addition, ACR-activated nuclear transcription factor E2-related factor 2 (Nrf2) and nuclear factor-κB (NF-κB) signaling pathways were also observed [6]. Moreover, analogous effects on inflammation parameters were reported in recent literature [11].
There is increasing attention to the role of oxidative stress in the pathogenesis of osteoporosis, in the context of several others classically known risk factors such as early menopause, low weight, the use of corticosteroids, smoking [12]. Cervellati et al. for example, suggested that estrogen withdrawal, typical of menopause, might contribute to increased bone vulnerability, with an increased risk of osteoporosis development [13]. Moreover, recent literature proposed a shift from the “estrogen-centric” account of the pathogenesis of osteoporosis, to another model where oxidative stress was also involved. In 2016 a meta-analysis including 17 observational studies [14] suggested a significant association between oxidative stress and osteoporosis, with the disequilibrium of ROS and antioxidant system as contributors to functional and structural remodeling of the bone. In animal models, some recent studies reported different effects on compact and trabecular bone tissue to a single oral acrylamide dose administration, with loss of thickness in cortical bone, through an increase in oxidative stress and inflammation [15, 16]. However, data regarding the association between dietary acrylamide and fractures in human beings are still missing, whilst increasing literature has reported that to follow a healthy diet pattern was associated with a lower incidence of fractures [17]. Finally, osteoporosis is often associated with important sequelae (such as disability and sarcopenia) [18] that further underlines the need of stratifying patients according to relevant risk factors [19, 20].
Having this in mind, the aim of the present study was to investigate the relationship between baseline dietary acrylamide intake and fractures development, over 8 years of follow-up. It is hypothesized that people with a higher intake of acrylamide would be at higher risk of osteoporotic fractures.
Materials and methods
Data source and participants
Information from the Osteoarthritis Initiative (OAI) database (https://www.niams.nih.gov/grants-funding/funded-research/osteoarthritis-initiative) were used for the present analyses. Potential participants were included from 4 different sites in the United States of America (Baltimore, MD; Pittsburgh, PA; Pawtucket, RI; and Columbus, OH) between February 2004 and May 2006.
All participants provided written informed consent. The OAI study was given full ethical approval by the institutional review board of the OAI Coordinating Center, at the University of California in San Francisco. Note that because this analysis was based on publicly available data, local ethical approval was not required.
Inclusion and exclusion criteria
The participants of the OAI must meet one of the following criteria: (i) being overweight; (ii) had a previous knee injury or surgery; (iii) had knee pain during the past year; (iv) or had a parent or sibling who had a knee replacement. Conversely, they were excluded if they suffer on rheumatoid arthritis, joint replacements in both knees, unable to walk without assistance or unable to undergo magnetic resonance of the knees.
Exposure
Dietary acrylamide intake was obtained through a food frequency questionnaire (FFQ) recorded during the baseline visit of the OAI. Dietary pattern was analyzed with the use of a validated tool called the Block Brief 2000 food-frequency questionnaire. This food-frequency questionnaire contains a list of 70 items and is designed to assess usual food and beverage consumption over the past year. Frequency of consumption of the 70 foods was reported at 9 levels of intake from never to every day. In addition, there were 7 dietary behavior queries on food preparation methods and fat intake, 1 on fiber intake, and 13 on vitamin and mineral intakes [21]. The product yearly frequency by portions’ size, contained in the FFQ, was categorized into standard portions (median value) [22] and then grams. The presence of acrylamide in foods was estimated using 2015 data from the Food and Drugs Administration (FDA) website (https://www.fda.gov/food/chemicals/survey-data-acrylamide-food). The general limit of quantitation (LOQ) of the method used by the FDA is 10 ppb. The data for this work regarding dietary acrylamide were reported as μg consumed in one year by each subject. The participants were divided in tertiles according to their dietary acrylamide intake, i.e., < 3313, between 3314 and 10,180, and > 10,180 μg, respectively.
Outcomes
The presence of fractures at baseline and during follow-up was ascertained through the self-reported history of fractures at the most common sites, i.e., hip, spine and forearm. The primary outcome was considered the incidence of any fracture, the osteoporotic specific sites (hip, forearm, spine) were considered as secondary outcomes. The presence of fractures in the OAI was recorded, other than the first evaluation, after 1, 2, 3, 4, 6 and 8 years from baseline.
Covariates
In these analyses, several covariates were identified as potential confounding factors, based on the literature available regarding dietary patterns and osteoporosis fractures. These included: age; gender; calorie intake (in Kcal); body mass index (BMI); depressive symptoms assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) [23]; physical activity evaluated using the total score for the Physical Activity Scale for the Elderly scale (PASE) [24]; race; smoking habit; educational attainment level (college or higher vs. others); yearly income (< or ≥ $50,000 or missing data); the modified Charlson Comorbidity Index score [25]; use of bisphosphonates/parathyroid hormones analogues (anti-osteoporotic medications) or estrogens/testosterone for menopausal symptoms; the presence of osteoporotic fractures at baseline; and dietary fibers in fruits and vegetables; dietary intake of vitamin D, calcium, proteins; number of alcoholic drinks during a typical week.
Statistical analyses
Continuous variables were normally distributed according to the Kolmogorov–Smirnov test. Therefore, data were shown as means and standard deviation values (SD) for quantitative measures. Percentages were used for discrete variables. Levene’s test was used to test the homoscedasticity of variances and, if its assumption was violated, Welch’s ANOVA was used. P-values were calculated using the Jonckheere-Terpstra test [26] for continuous variables and the Mantel–Haenszel Chi-square test for categorical variables.
For assessing the relationship between dietary acrylamide intake and the onset of fractures during follow-up, a Cox’s regression analysis was done. The incidence of fractures during the follow-up, evaluated at visit number 1, 3, 5, 6, and 8 was graphically reported using survival curves. Deceased people were censored. Dietary acrylamide was categorized into tertiles, with Tertile 1 (the lowest intake) representing the reference group. Multi-collinearity among covariates, cited before, was assessed using variance inflation factor (VIF) [27], taking a cut-off of 2 as the criterion for exclusion, even if no covariates met this criterion. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were reported to estimate the presence and the strength of the associations between dietary acrylamide intake and incident osteoporotic fractures. P values for trend were calculated across dietary acrylamide tertiles using the Wald test, based on a score derived from the median value of each baseline tertile [28].
Some sensitivity analyses were conducted for showing the interaction between dietary acrylamide intake and some selected variables (i.e., age below or more/equal than 65 years, overweight/obese (≥ 25 kg/m2) vs. normal weight (18.5 kg/m2 < BMI < 25 kg/m2), yearly income, gender, race, education, smoking habits, presence of fractures at the baseline, use of anti-osteoporotic medications) in the association with incident fractures, only presence of fractures at the baseline and use of anti-osteoporotic medications emerged as effect modifiers of the associations examined (p < 0.0001 for the interaction).
All analyses were performed using the SPSS 20.0 for Windows (SPSS Inc., Chicago, Illinois). All statistical tests were two-tailed and statistical significance was assumed for a p-value < 0.05.
Results
Study participants
In 4796 initially included individuals, 243 reported < than 500 or > 5000 kcal (not reliable calorie intake) or did not have any information regarding FFQ and 117 were lost at follow-up (i.e. did not have information regarding fractures during the follow-up period), leaving 4436 participants included for this research. The 360 people not included were significantly older and more frequently females than people included.
Baseline analyses
Altogether, 4436 participants (of them 2578 women) with a mean age of 61.3 ± 9.1 (range: 45–79) years were included in the analysis. The mean acrylamide intake, by year, was 13,985 ± 23,863 (range: 0–364,438) μg (mean 38.3 μg/day).
The baseline characteristics, by dietary acrylamide intake divided into tertiles, are shown in Table 1. People having a greater dietary acrylamide intake (T3) were significantly younger, more frequently males, less educated, had a significant higher calorie intake and were more frequently obese and sedentary than those introducing less acrylamide with their diet (T1) (Table 1). People introducing more acrylamide with their diet reported a significantly higher consumption of proteins and fibers, derived from vegetables, as well as calcium. People with the highest tertiles of dietary acrylamide intake used less frequently anti-osteoporotic medications (7.0 in T3 vs. 19.0% in T1, p < 0.0001) and they reported more frequently osteoporotic fractures (19.8 in T3 vs. 16.3% in T1, p = 0.01) (Table 1).
Follow-up analyses and incident fractures onset
During eight years of follow-up, 789 osteoporotic fractures were observed. As shown in Table 2, people with higher dietary acrylamide intake reported a significant higher incidence of fractures compared to those with lower dietary acrylamide intake (34, 95% CI 30–38 in T3 vs. 25, 95% CI 22–29 in T1).
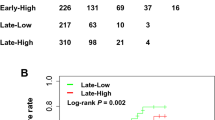
In multivariable analyses, after taking into account 18 different potential confounders at baseline, compared to participants with lower acrylamide intake (T1), those with a higher acrylamide intake (T3) reported a significant higher risk of any fracture (HR = 1.37; 95% CI 1.12–1.68; p = 0.002) (p for trend = 0.009). Similar results were observed taking, as outcomes, specific-site fractures, including forearm fracture (HR = 1.73; 95% CI 1.09–2.77) (p for trend = 0.04), spine fracture (HR = 2.21; 95% CI 1.14–4.31; p for trend = 0.04), and hip fracture (HR = 4.09; 95% CI 1.29–12.96; p for trend = 0.046) (Table 2).
In sensitivity analyses, it was found that the association between dietary acrylamide intake and incident fractures was stronger in people with the presence of an osteoporotic fracture at the baseline (T3: HR = 1.84; 95% CI 1.18–2.22, p = 0.002) (p for interaction < 0.0001) and in those not taking any anti-osteoporotic medication (T3: HR = 1.36; 95% CI 1.14–1.84; p = 0.001) (p for interaction < 0.0001). The p for the interaction sex by baseline dietary acrylamide intake was 0.48, indicating no difference between males and females in reporting fractures related to this factor.
Discussion
In the present study, involving a large cohort of adults with or at high risk of knee OA residing in North America, it was found that people with higher dietary acrylamide intake have an increased risk of osteoporotic fractures, over eight years of follow-up. This finding remained unaltered after adjustment for several potential confounders and when considering site-specific fractures. Finally, effects were stronger in people having already had a diagnosis of osteoporosis at baseline and in those not taking any medication for this condition.
In a review including 101 studies and investigating the dietary intake of acrylamide, the authors found mean/median values of acrylamide in North American people lower than those found in the OAI, probably because in the review general populations are reported whilst in the OAI obese/overweight people are included [2]. At baseline people having a higher dietary acrylamide intake were significantly younger, more frequently males, less educated, had a significantly higher calorie intake and were more frequently obese and sedentary than those introducing less acrylamide with their diet. These findings were expected, since in this study a high acrylamide intake reflects a propensity to an unhealthy diet, rich in fried and roasted food, common in North American obese people and in younger populations [29,30,31]. Moreover, it has been reported that North American women more frequently consume healthy food such as vegetables and fruits than men [32] and that there is a strong consistent relationship between low socio-economic factors, as low scholar education, in early life and increased obesity in adulthood [33]. Furthermore, diets rich in fruit and vegetables have been shown to decrease the risk of the incidence of hip fractures [34, 35].
To the best of our knowledge, this is the only study evaluating the relationship between the dietary intake of acrylamide and osteoporotic fractures in human beings. There are only two studies in animals that evaluated the effects of an acute oral administration of acrylamide on bone microstructure analysis, finding a significant loss of thickness in femoral cortical bone of mice after oral acrylamide exposure [15, 16]. More recently, another in the animal study reported that the exposure to acrylamide resulted in skeletal structure malformation in zebrafish and rat embryos and that the toxicity of acrylamide might translate to the next generation [36]. In particular, the term cortical osteoporosis includes mechanically inappropriate cortical thinning as well as excessive porosity and is almost certainly a relevant factor in hip fracture in older people [37, 38]. In normal conditions, cortical bone tissue deforms under a mechanical load and then regains its previous anatomical form, the elasticity of cortical bone tissue is a measure of resistance to deformation [39]. Another important point that future research should verify is that the association between dietary acrylamide and incident fractures is stronger in people already having osteoporosis. With the data available so far, one can only give a tentative suggestion in limiting the introduction of fried foods in those with osteoporosis as this may result in a higher vulnerability to fractures.
From a pathophysiological point of view, it is possible that ROS could be the link between dietary acrylamide intake and osteoporosis. As already pointed out, acrylamide exposure has been shown to lead to oxidative stress characterized by a significant increase in reactive oxygen species [10]. In a meta-analysis on the association between oxidative stress and osteoporosis, increased levels of homocysteine and nitric oxide in osteoporotic participants and decreased levels of folates and total antioxidant power, along with the lower activity of superoxide dismutase and glutathione peroxidase, were reported suggesting a significant association between oxidative stress and osteoporosis [14].
The present findings have potentially important clinical implications, not only limited to acrylamide intake and osteoporotic fractures. Major food sources of acrylamide are deep fried and roasted food, commonly consumed as a part of an unhealthy diet. Conversely, a diet with high consumption of vegetables has been related to have a protective effect against osteoporosis and fractures [40]. However, it should be noted that vegetarians (in particular vegans) are exposed to a higher risk of fractures than omnivores, possibly due to a lack of other important nutrients, including calcium and protein [41, 42].
Finally, it is important to discuss how to limit dietary acrylamide intake. The first, obvious, intervention is to limit those foods rich in acrylamide such as fried fries and the other foods undergoing the Maillard reaction [43]. Another method to limit the production of dietary acrylamide is adding some nutrients, such as vitamins, minerals, amino acids, and in cooking oils, even if these findings are still exploratory [43].
Findings from this study should be interpreted in light of its limitations. First, dietary acrylamide was assessed only at baseline; therefore, it was not possible to assess whether changes in acrylamide consumption over time can modify the results. In this regard, we also note that its estimation can be difficult using the FFQ or other traditional nutritional tools. In particular, even if FFQ is the most used tool in nutritional epidemiology, the assessment of dietary acrylamide using this tool is not validated, and therefore our results should be treated with caution. Second, the information regarding fractures was self-reported in the OAI and we do not have specific information regarding if a fracture was due to osteoporosis or other causes. In this regard, some studies showed that for clinical fractures the accuracy of self-reported fractures is accurate and similar to radiological records, but probably there is an underestimation of some non-clinical fractures, especially vertebral ones [44, 45]. Third, no data about bone mineral density, renal function and vitamin D levels are available and this could introduce another bias in the findings. Fourth, the OAI selectively oversamples individuals who are obese. Therefore, it is likely that the simple adjustment for BMI might be unable to remove residual confounding attributable to adiposity in this specific population. Fifth, the small number of cases in the site-specific fracture, such as 4 cases in T3 for hip fracture, indicate to take cautiously the findings regarding specific-sites fractures. Furthermore, the OAI included only participants with knee osteoarthritis or at high risk of this condition, likely introducing a selection bias, and limiting the generalizability of results. Finally, no information on the menopausal status of the women in the study was available, therefore the effect of menopausal status (and associated hormone replacement therapies) are unknown.
In conclusion, higher dietary acrylamide intake was significantly associated with a higher risk of osteoporotic fractures, also after accounting for potential confounders, suggesting a role for this food contaminant as a possible risk factor for osteoporosis. Overall, people with introducing more acrylamide with their diet reported a significantly higher risk of any site and specific site (forearm, hip, vertebral) fractures. Our study further underlines the importance of consuming low amounts of acrylamide with a diet that seems associated with several negative outcomes in human beings including cancer and now osteoporotic fractures.
References
Stadler RH, Blank I, Varga N et al (2002) Acrylamide from Maillard reaction products. Nature 419:449–450
Timmermann CAG, Mølck SS, Kadawathagedara M et al (2021) A review of dietary intake of acrylamide in humans. Toxics 9:155
Sun G, Qu S, Wang S et al (2018) Taurine attenuates acrylamide-induced axonal and myelinated damage through the Akt/GSK3β-dependent pathway. Int J Immunopathol Pharmacol 32:2058738418805322
Elmore JS, Koutsidis G, Dodson AT et al (2005) The effect of cooking on acrylamide and its precursors in potato, wheat and rye. Chemistry and safety of acrylamide in food. Springer, Berlin
Törnqvist M (2005) Acrylamide in food: the discovery and its implications. Chem Saf Acrylamide Food. https://doi.org/10.1007/0-387-24980-X_1
Benisi-Kohansal S, Salari-Moghaddam A, Rohani ZS et al (2021) Dietary acrylamide intake and risk of women’s cancers: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr 126:1355–1363
Ikeda S, Sobue T, Kitamura T et al (2021) Dietary acrylamide intake and the risks of renal cell, prostate, and bladder cancers: a Japan public health center-based prospective study. Nutrients 13:780
Hogervorst JG, Saenen ND, Nawrot TS (2021) Gestational acrylamide exposure and biomarkers of fetal growth: probing the mechanism underlying the association between acrylamide and reduced fetal growth. Environ Int 155:106668
Mikeš O, Brantsæter AL, Knutsen HK et al (2021) Dietary patterns and birth outcomes in the ELSPAC pregnancy cohort. J Epidemiol Commun Health. https://doi.org/10.1136/jech-2020-215716
Pan X, Wu X, Yan D et al (2018) Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol Lett 288:55–64
Acaroz U, Ince S, Arslan-Acaroz D et al (2018) The ameliorative effects of boron against acrylamide-induced oxidative stress, inflammatory response, and metabolic changes in rats. Food Chem Toxicol 118:745–752
McCloskey EV, Johansson H, Oden A et al (2009) From relative risk to absolute fracture risk calculation: the FRAX algorithm. Curr Osteoporos Rep 7:77–83
Cervellati C, Bonaccorsi G, Cremonini E et al (2013) Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin Chem Lab Med 51:333–338
Zhou Q, Zhu L, Zhang D et al (2016) Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Mark. https://doi.org/10.1155/2016/7067984
Sarocka A, Kovacova V, Omelka R et al (2019) Single and simultaneous effects of acrylamide and ethanol on bone microstructure of mice after one remodeling cycle. BMC Pharmacol Toxicol 20:1–9
Sarocka A, Babosova R, Kovacova V et al (2017) Acrylamide-induced changes in femoral bone microstructure of mice. Physiol Res 66:1067–1071
Fabiani R, Naldini G, Chiavarini M (2019) Dietary patterns in relation to low bone mineral density and fracture risk: a systematic review and meta-analysis. Adv Nutr 10:219–236
Borgström F, Karlsson L, Ortsäter G et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15:1–21
de Sire A, Invernizzi M, Baricich A et al (2021) Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: a patient-tailored plan from orthopaedics to rehabilitation. World J Orthop 12:456
Pasco JA, Mohebbi M, Tembo MC et al (2020) Repurposing a fracture risk calculator (FRAX) as a screening tool for women at risk for sarcopenia. Osteoporos Int 31:1389–1394
Block G, Hartman AM, Naughton D (1990) A reduced dietary questionnaire: development and validation. Epidemiology 1:58–64
Pfrimer K, Sartorelli DS, Rosa FT et al (2013) Calibration of the food list and portion sizes of a food frequency questionnaire applied to free-living elderly people. Nutrition 29:760–764
Lewinsohn PM, Seeley JR, Roberts RE et al (1997) Center for epidemiologic studies depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12:277
Washburn RA, McAuley E, Katula J et al (1999) The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol 52:643–651
Katz JN, Chang LC, Sangha O et al (1996) Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73–84
Jonckheere AR (1954) A distribution-free k-sample test against ordered alternatives. Biometrika 41:133–145
Miles J (2014) Tolerance and variance inflation factor. Wiley StatsRef Statistics Reference Online, New Jersy
Sommer S, Huggins RM (1996) Variables selection using the Wald test and a robust CP. J Roy Stat Soc 45:15–29
Mayne SL, Virudachalam S, Fiks AG (2020) Clustering of unhealthy behaviors in a nationally representative sample of US children and adolescents. Prev Med 130:105892
Qi Q, Chu AY, Kang JH et al (2014) Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ. https://doi.org/10.1136/bmj.g1610
Besdine RW, Wetle TF (2010) Improving health for elderly people: an international health promotion and disease prevention agenda. Aging Clin Exp Res 22:219–230
Lee-Kwan SH, Moore LV, Blanck HM et al (2017) Disparities in state-specific adult fruit and vegetable consumption—United States, 2015. MMWR Morb Mortal Wkly Rep 66:1241
Witkam R, Gwinnutt JM, Humphreys J et al (2021) Do associations between education and obesity vary depending on the measure of obesity used? A systematic literature review and meta-analysis. SSM Popul Health 15:100884
Malmir H, Saneei P, Larijani B et al (2018) Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: a systematic review and meta-analysis of observational studies. Eur J Nutr 57:2147–2160
Barnsley J, Buckland G, Chan P et al (2021) Pathophysiology and treatment of osteoporosis: challenges for clinical practice in older people. Aging Clin Exp Res 33:759–773
Zhu F, Wang J, Jiao J et al (2021) Exposure to acrylamide induces skeletal developmental toxicity in zebrafish and rat embryos. Environ Pollut 271:116395
Power J, Loveridge N, Lyon A et al (2003) Bone remodeling at the endocortical surface of the human femoral neck: a mechanism for regional cortical thinning in cases of hip fracture. J Bone Miner Res 18:1775–1780
Celi M, Rao C, Scialdoni A et al (2013) Bone mineral density evaluation in osteoporosis: why yes and why not? Aging Clin Exp Res 25:47–49
Reeve J (2017) Role of cortical bone in hip fracture. BoneKEy Rep. https://doi.org/10.1038/bonekey.2016.82
Li Y, Luo H, Zhao K (2016) Increased intake of vegetables, but not fruits, may be associated with reduced risk of hip fracture: a meta-analysis. Sci Rep 6:1–8
Iguacel I, Miguel-Berges ML, Gómez-Bruton A et al (2019) Veganism, vegetarianism, bone mineral density, and fracture risk: a systematic review and meta-analysis. Nutr Rev 77:1–18
Veronese N, Reginster J-Y (2019) The effects of calorie restriction, intermittent fasting and vegetarian diets on bone health. Aging Clin Exp Res 31:753–758
Rifai L, Saleh FA (2020) A review on acrylamide in food: occurrence, toxicity, and mitigation strategies. Int J Toxicol 39:93–102
Øyen J, Torstveit MK, Sundgot-Borgen J (2009) Self-reported versus diagnosed stress fractures in Norwegian female elite athletes. J Sports Sci Med 8:130
Ivers RQ, Cumming RG, Mitchell P et al (2002) The accuracy of self-reported fractures in older people. J Clin Epidemiol 55:452–457
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. Publication costs are supported by the FFR2021 fund of the University of Palermo assigned to Prof. Mario Barbagallo and Nicola Veronese. The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rizzoli reports personal fees from has received fees for advisory board or lectures from Abiogen, Amgen, CNIEL, Danone, Echolight, Effryx, EMF, Mithra, Mylan, Nestlé, ObsEva, Radius Health, Rejuvenate, Sandoz and Theramex; Dr. Reginster reports grants and personal fees from IBSA-GENEVRIER, grants and personal fees from MYLAN, grants and personal fees from RADIUS HEALTH, personal fees from PIERRE FABRE, grants and personal fees from CNIEL, personal fees from DAIRY RESEARCH COUNCIL (DRC); Professor Cyrus Cooper has received lecture fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Medtronic, Merck, Nestlé, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB; Dr. Veronese reports personal fees from Mylan and Fidia. These authors reported that these grants and personal fees are outside the submitted work. The other authors have nothing to disclose.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the OAI Coordinating Center, at University of California in San Francisco.
Consent to participate
Informed consent was obtained from all subjects involved in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Veronese, N., Bolzetta, F., Cacco, C. et al. Dietary acrylamide and incident osteoporotic fractures: an 8-year prospective cohort study. Aging Clin Exp Res 34, 2441–2448 (2022). https://doi.org/10.1007/s40520-022-02214-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02214-9