Abstract
Background
Myosteatosis, skeletal muscle fat infiltration, is associated with inflammation and fibrosis. The age-related increase of myosteatosis is an important characteristic of sarcopenia and contributes to fragility.
Aims
To investigate the impact of healthy aging on intermuscular adipose tissue (IMAT) and muscle fat fraction (FF) in the thigh and the paraspinal muscles in males.
Methods
In 54 healthy males (age 20–70), all active hobby golfers, magnetic resonance imaging was performed to determine volume of IMAT, volume of muscle tissue (MT) and of percentage of FF.
Results
Between ages 20–70, at the thigh, IMAT/MT volume and MT FF increased annually by 2.9% and 1.3%, respectively. At the psoas IMAT/Psoas volume did not change with age. MT FF increased by 1.5% annually. At the erector spinae IMAT/Erector volume decreased by 0.3% and MT FF increased by 2.8% annually.
Discussion
With increasing age, in males, thigh muscle atrophied, muscle tissue was partly replaced by adipose tissue and remaining muscle tissue also contained more fat. Similar effects were observed in the erector spinae. The psoas muscle did not atrophy, although MT FF also increased with age. Overall correlations with age were weak to moderate with higher correlations observed in the paraspinal muscles.
Conclusions
Age-related increases of muscle fat infiltration were observed in the thigh and in the spine. Muscle atrophy did not occur in the psoas. In cross-sectional studies, an adjustment of volumetric parameters by muscle volume is advisable when comparing age-dependent results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the latest definition of the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), muscle quantity and quality are part of the sarcopenia case-finding algorithm [1]. A decrease in muscle volume is associated with a decrease of muscle strength and consequently of physical function [2]. However, muscle strength is not just a function of muscle volume. In older adults, the age-related decrease of muscle strength is about three times as high as the decrease of appendicular lean mass measured by dual X-ray absorptiometry (DXA), which is a surrogate of muscle mass [3]. Myosteatosis (skeletal muscle fat infiltration) is another important component of mobility and muscle strength [4,5,6,7,8], which is highly associated with muscle fat fraction [9, 10].
Myosteatosis of the thigh for example, includes the adipose tissue and lipids beneath the fascia lata. For the purpose of this study, two components are differentiated. The first one is IMAT (intermuscular adipose tissue) defined as "visible storage of lipids in adipocytes located between the muscle fibers (also termed intramuscular fat) and also between muscle groups (literally intermuscular)” [4, 11]. In T1-weighted MR (magnetic resonance) images the signal intensity of IMAT appears white whereas the signal intensity of the complement of IMAT, which is termed muscle tissue (MT) appears dark. Traditionally, radiologists assessed muscle fat infiltration by semi quantitatively grading IMAT of the thigh or calf using CT or MR images [12]. Intramuscular fat may be deposited in two distinct compartments, either as intramyocellular lipids (IMCLs) accumulated in the cytoplasm of myocytes or as extramyocellular lipids (EMCLs) in interstitial, intramyofascial adipocytes.
The second component of myosteatosis is not detectable (invisible) in T1-weighted MR images. This component consists of small aggregates of adipocytes between muscle bundles and intramyocellular lipids. However, their combined contribution to myosteatosis can be quantified indirectly from MRI Dixon sequences as fat fraction (FF) of MT [13, 14]. A separation of IMCLs and EMCLs can be achieved by MR spectroscopy but not by MR imaging [15]. An accurate separation of IMAT from muscle tissue of the thigh or calf requires a segmentation of the deep fascia lata (FL) [16], a highly complex network of extracellular matrix and cells of mesenchymal and neural origin [17].
Compared to young healthy subjects, in sarcopenic subjects IMAT volume of lower extremity tissues is increased [18] and in older adults IMAT is associated with decreased mobility [19]. A small Japanese study also reported that between 20 and 70 years the increase of intramuscular adipose tissue volume normalized to muscle volume was larger than the decrease of muscle cross-sectional area normalized to body weight [20]. IMAT was also higher in frail versus non-frail individuals and this was significantly associated with inflammation as analyzed by muscular IL-6 expression [21].
We demonstrated a larger effect of a high intensity training on IMAT than on muscle tissue fat fraction [22] Bruseghini et al. also reported a decrease of IMAT volume after high intensity training in a small group of elderly men [23]. Recently Farrow et al. [24], Hogrel et al. [25] and Yoon et al. [26] using MRI and Delmonico et al. [27] using CT showed age-related increases of fat fraction of the complete thigh muscle ensemble [25,26,27] and also of individual thigh muscles [24]. However, none of the three studies performed a FL segmentation and IMAT volume was not determined. Also differences between male and female subjects were not presented. An age-related increase of IMAT of the quadriceps muscle of elderly inpatients (> 65 years) was also reported using ultrasound [28]. In abdominal CT images of Chinese women, Peng et al. [29] showed an age-related increase of IMAT and a decrease of muscle cross-sectional area of the paraspinal muscles. An age-related increase of paraspinal FF has also been detected by MRI [30].
To better understand mechanisms of myosteatosis, it is important to separate disease-related effects from those of healthy aging and to investigate the impact of healthy aging on the different components of myosteatosis. Age-related changes must be determined separately for males and females and for different ethnicities—a long to do list. In this study, we explored age-related changes of IMAT volume and muscle tissue fat fraction of the thigh and the paraspinal muscles in active healthy white males.
Methods
In this study, baseline data of the Franconian EMS and Golf (FREMGO) study [31] were used. FREMGO has been approved by the Friedrich-Alexander University Erlangen-Nürnberg (FAU) Ethics Committee (number 377_19b) and was registered under ClinicalTrials.gov: NCT04264416. The study fully complied with the Helsinki Declaration “Ethical Principles for Medical Research Involving Human Subjects” [32]. All study participants gave their written informed consent after having received detailed information.
Participants
The baseline data of FREMGO consisted of 54 normal healthy German males of age 20–70. All were active hobby or amateur golfers (handicaps: 8–54) of local clubs in Northern Bavaria. None of them carried out athletic sportive activities. Resistance exercise for more than 60 min/week during the 12 months prior to baseline assessments was an exclusion criterion.
Assessments
Assessments included body composition, MRI of the thigh and paraspinal muscles and a standardized questionnaire for demographic parameters, pain, diseases, injuries and surgery. Participants were requested to refrain from intense physical activity and exercise 48-h pre-testing. Assessments were always performed at the same time of the day (± 90 min), in the same order and by the same researcher using identically calibrated devices.
Body composition
Body height was assessed by a stadiometer. Body composition and appendicular skeletal muscle mass (ASMM) were measured using a direct-segmental, multi-frequency bio impedance analysis device (DSM-BIA, InBody770, Seoul, Korea).
Magnetic resonance imaging of the thigh
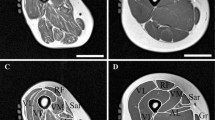
MRI acquisitions were performed on a 3T scanner (MAGNETOM Prisma Fit, Siemens Healthineers AG, Erlangen, Germany) using an 18-channel body surface coil. The protocol included a T1-weighted Turbo Spin Echo and a 6-point Dixon Gradient Echo sequence (Fig. 1a) to determine fat fraction (FF) [14]. For theT1 weighted sequence the following parameters were used: voxel size 0.5 × 0.5 × 3.0 mm3, 34 slices, matrix size 512 × 512, TR 844 ms, echo time (TE) 14 ms, bandwidth 488 Hz/px, acquisition time 2:54 min. For the 6pt Dixon sequence the following parameters were used: voxel size 1.0 × 1.0 × 3.0 mm3, 36 slices, matrix size 256 × 256, TR 14.0 ms, TEs 1.90, 3.73, 5.56, 7.39, 9.22 and 11.05 ms, bandwidt 850 Hz/px, flip angle 6°, acquisition time 1:17 min. Intensities of the Dixon FF images ranged from 0 to 1000 corresponding to a FF of 0–100%.
a MRI of the thigh. Left: T1 weighted sequence with segmented fascia lata; center: Dixon fat fraction image; right: separation of IMAT (yellow) and muscle tissue (red). b MRI of paraspinal muscles. Left: T1 weighted sequence with segmented muscles; center: Dixon fat fraction image; right: separation of IMAT (yellow) and muscle tissue (dark)
Magnetic resonance imaging of the spine
At the spine (Fig. 1b) axial slices with a thickness of 3 mm were acquired from mid L1 to mid S1. For theT1 weighted sequence the following parameters were used: voxel size 0.4 × 0.4 × 3.0 mm3, 44 slices, matrix size 512 × 336, TR 540 ms, echo time (TE) 11 ms, bandwidth 227 Hz/px, acquisition time 4:57 min. For the 6pt Dixon sequence the following parameters were used: voxel size 0.9 × 0.9 × 3.0 mm3, 36 slices, matrix size 288 × 168, TR 17.10 ms, TEs 1.90, 3.71, 7.20, 10.68, 12.49 and 14.30 ms, bandwidth 710 Hz/px, flip angle 4°, acquisition time 1:46 min.
Image analysis
Image analysis was performed by a trained technician using MIAF (Medical Image Analysis Framework, IMP). The analysis started with the segmentation of the fascia lata in the T1 images (Fig. 1a). Segmentation masks generated in the T1 images were then registered to the corresponding Dixon FF images as described before [16]. Muscle and adipose tissue were automatically separated in the Dixon FF images (Fig. 1a) based on the local minimum of the patient-specific histogram of all voxels within the fascia. The actual threshold used for the separation of muscle and adipose tissue was the FF at 50% below the minimum. This approach is similar to the one used by Rossi et al. [33, 34] who used a threshold of 20% below the minimum in T1 weighted images. Rossi et al. did not obtain Dixon images. Volume of the fascia (intra fascia volume) and of IMAT were measured. In addition, FF of muscle tissue (MT), the complement of IMAT within the FL was determined. Individual muscles were not distinguished. For this analysis technique inter- and intra-operator precision errors (root mean square % coefficients of variation) of lower than 1% for IMAT volume and FF have been reported earlier [16].
The same workflow was used in the spine (Fig. 1b) but only one slice at level L2 was analyzed. Right and left psoas and erector spinae muscles were segmented manually using ImageJ (https://imagej.nih.gov/ij/). The multifidus muscle was included in the erector spinae region of interest. Separate results for IMAT and muscle tissue FF were obtained for the psoas and erector spinae muscles as average values of left and right muscles.
Statistical procedures
Age-related changes were fitted by polynomial regressions using linear and quadratic models. Annual %change was calculated from slope and intercept and the %CV value was calculated from the standard error of the estimate (SEE) of the linear regression and the corresponding mean y value. A P value of < 0.05 was considered significant. All data analyses were performed using SPSS (IBM SPSS Statistics, Version 26.0. Armonk, NY: IBM Corp).
Results
Age-related changes of the thigh
MRI scans of the thigh were available from 48 and of the spine from 44 of the 54 subjects included in FREMGO. One MRI scan of the thigh and one of the spine were not analyzable because of motion artifacts.
Age-related changes of body mass index (BMI), height and weight of the study population did not differ from corresponding changes of the German normal male population [35] shown in Fig S1.
Tables 1 and 2 show results of linear regressions with age of the thigh and spine, respectively. Annual percent changes relative to age 20 are shown if regressions were significant. Tables S1 and S2 show results of quadratic regressions. In these tables, percent changes from 20 to 40 years relative to age 20 and from 40 to 70 years relative to age 40 are shown if regressions were significant.
FL and MT volume showed a quadratic dependence of age (Fig. 2) with an increase of 12% for FL volume and 8% for MT volume between age 20 and 40 followed by a decline of 29% for FL volume and 25% for MT volume between age 40 and 70. IMAT volume increased linearly by 1.9% per year. The age-related increase of MT FF could also be approximated by a linear regression with an annual increase of 1.3%.
Correlations of FL, IMAT and MT volume with age were weak (R2 < 0.25). Normalization of IMAT to MT volume increased the annual percentage changes from 1.9% for IMAT alone to 2.9%. In parallel, the correlation with age increased to R2 = 0.38. The 1.6% annual increase of FF of the FL was higher than of MT FF (1.3%) because it also reflected the relative increase of IMAT with age. Normalization of IMAT volume or MT FF to BMI or ASMM measured by bio impedance had little effect. If linear regressions were significant, quadratic regressions were not superior in terms of R2, P and SEE.
Age-related changes of the psoas
Most regressions with age were not significant. Psoas volume and MT volume showed an almost identical quadratic dependence of age (Fig. 3a) with an increase of 13% for FL volume and 14% for MT volume between age 20 and 40 followed by a decline of 35% for FL volume and 37% for MT volume between age 40 and age 70. Consequently, MT/psoas volume and IMAT volume that constituted only 6% of the psoas volume did not significantly change with age. However, there was a significant 1.5% annual increase in MT FF of the psoas.
a Age-related changes of FL, IMAT and MT volume and MT FF at the psoas muscle. Linear and quadratic fits are shown as solid and dashed lines, respectively. b Age-related changes of FL, IMAT and MT volume and MT FF at the erector spinae muscle. Linear and quadratic fits are shown as solid and dashed lines, respectively
Age-related changes of the erector spinae
Erector spinae volume (Fig. 3b) did not significantly decrease with age. In contrast, all other volume and FF measurements showed significant linear increases with age, most notably a 12.6% annual increase of IMAT volume, which resulted in a 20% annual increase of IMAT/erector volume. Linear regressions were highly significant but for the erector, the quadratic regressions resulted in better fits in particular at lower age (Fig. 3b). R2 values were slightly higher and the fits indicate relative stability of the parameters up to age 40. Thus for the erector annual % changes relative to age 20 shown in Table 2 must be interpreted with caution as the linear fits resulted in very low values at age 20. Consequently, the annual increase of IMAT / MT volume was 400%. The quadratic regression shows an increase of 14% during the first 20 years followed by a 200% increase between age 40 and 70.
Discussion
In this study, we determined age-related changes of IMAT and muscle tissue fat fraction of the mid-thigh and paraspinal muscles in active healthy males between age 20 and 70. In the thigh, IMAT and muscle tissue fat fraction increased with age but interestingly the dependence of IMAT on age only became significant after adjustment by fascia volume. An increase in IMAT volume combined with a decrease in fascia volume implies a decrease of muscle tissue volume with age. Thus, there was a combination of age-related increase in IMAT volume with concurrent muscle atrophy. In addition, the fat fraction of the remaining muscle tissue further increased. Thus with increasing age, thigh muscle atrophied, muscle tissue was partly replaced by adipose tissue and remaining muscle tissue also contained more fat.
In the spine, similar effects were observed in the erector spinae, in contrast to the psoas muscle, where IMAT volume and muscle tissue volume did not change with age. The psoas muscle as an anatomical structure did not atrophy, however, muscle tissue fat fraction also increased with age. Correlations with age were weak in the thigh and moderate in the paraspinal muscles. Independent of anatomy, correlations of muscle tissue fat fraction with age were higher than for IMAT volume.
Comparison of our results with previously published data is difficult because in many publications the measurement of IMAT and FF is not clearly described. There is also an ongoing discrepancy in the definition of IMAT. We followed the majority of earlier publications [4, 11] defining IMAT as the combination of extracellular adipose tissue between muscle groups and of larger adipocyte agglomerations within muscle, which originated in the early radiological assessment of muscle fat infiltration based on T1-weighted images.
In contrast, a recent Interdisciplinary Workshop at the National Institute on Aging stated that myosteatosis included three components "(a) intermuscular adipose tissue (IMAT), the extra-myocellular adipose tissue found beneath the fascia and in-between muscle groups; (b) intramuscular adipose tissue, the extramyocellular adipose tissue found within an individual muscle; and (c) intramyocellular lipids (IMCL)” [36]. According to this definition, IMAT does not include the extracellular adipose tissue found within an individual muscle. Obviously, standardization of terminology is needed but more importantly, it is still not entirely clear what compartment is most relevant from a medical perspective.
In vivo, IMCL can be assessed with MR spectroscopy [15] but intramuscular adipose tissue cannot be separated from intramyocellular lipids by in vivo imaging due to limited spatial resolution, thus combining larger detectable agglomerations of extracellular adipose with IMAT and separating it from adipose tissue of the remaining muscle tissue seems to be straightforward for in vivo muscle MRI. Also precision errors for MR spectroscopy are much higher than for MRI [13]. The reality is even more complex because in many studies of the thigh the facia lata was not segmented. Instead a tight contour around the muscle ensemble was used. The effect may be small in younger subjects with usually little adipose tissue directly beneath the FL. However, in elderly subjects, without a correct segmentation of the fascia lata IMAT volume will be underestimated regardless of the IMAT definition.
The missing differentiation of the FF of MT in IMCL versus extramyocellular lipids (EMCL), the fat content of interstitial, intramyofascial adipocytes is an important limitation of in vivo MRI. For example, a recent study on back pain using MR spectroscopy showed that EMCL content of the psoas correlated with age, but IMCL content did not [37]. Adipocytes result from differentiation of resident mesenchymal precursors such as Fibro-Adipogenic Precursor (FAP) cells and their expansion. FAP cells can differentiate towards the fibrocyte and the adipocyte pathways [38] and have been shown to be crucial components of muscle regeneration [39]. However, they may also be an origin of fibrotic and fatty infiltration in pathology [40]. ICMLs, lipid droplets (LD) as ubiquitous cellular organelles, provide sources of energy and contribute to the effects of exercise on hypertrophy but also to many other cellular functions like the constitution of the cytoskeletal system and mitochondrial activity [41]. For example, LDs can accumulate in muscle cells in well-trained athletes as a source of energy, but pathologic accumulation of LDs of somewhat different size is associated with insulin resistance, a phenomenon that has been coined ‘the athletes paradox’ [42,43,44].
Compared to other publications our study has several advantages but also further limitations. We reported data on thigh and paraspinal muscles, but we only included men. The number of less than 50 subjects with available MR images was relatively small but this was also the case for most other studies reporting age-related changes of IMAT or FF. We reported a large variety of combinations of absolute and % values for IMAT and FF. In addition, the age distribution of our cohort was more homogenous than in other studies, in which often just younger and older age groups were compared. However, subjects older than 70 years were not available in our cohort and in particular, the quadratic fits may be inaccurate for age > 65.
For example, Buford et al. [18] showed a 30% increase in IMAT volume of the thigh between elderly (76.4 ± 5.7 years) and younger (23.8 ± 3.9 years) male and female subjects whereas our results in males showed a 60% increase. Part of the discrepancy can probably be attributed to the segmentation of the fascia lata, but exact details were not reported. Farrow et al. [24] compared fat fraction of two thigh muscles among three age groups (26 ± 8 years, 49 ± 19 years, and 79 ± 5 years) of mixed gender. FF of the hamstrings increased with age from 3.4 to 5.6 and 9.5%, i.e., by a factor of 1.6 of the middle aged versus the younger and by a factor of 2.7 of the old versus the young group with similar relative increases in the quadriceps. Our results for MT FF of the whole thigh showed a smaller increase from 3.7% at age 25 to 4.8% at age 50 and 6.1% at age 80 but as discussed above our FF analysis excluded larger agglomerations of intramuscular adipose tissue.
Farrow’s FF analysis was based on segmentation of individual muscles. Typically, this is performed manually and restricted to single slices of the MRI dataset, whereas for the thigh we applied a full 3D analysis and determined FF not in an entire muscle but in muscle tissue. Differences will be small in younger subjects with little intramuscular adipose tissue (Fig. 1a) but in elderly people FF of the complete muscle as determined by Farrow et al. is expected to be higher than FF of the muscle tissue.
Hogrel et al. [25] also performed a segmentation of individual muscles similar to the study from Farrow et al. and compared muscle volume and FF between young (20–30 years) and older (70–80 years) males and females. Similar to our results they also reported an age-related muscle atrophy and a concurrent increase in muscle FF. Decrease in total thigh muscle volume was 22% in men and 21% in women and increase of total thigh muscle FF was 87% in men (compared to 60% for muscle tissue FF in our study) and 68% in women. As a disadvantage, neither Farrow et al. nor Hogel et al. reported IMAT results.
A more comprehensive study was performed by Yoon et al. [26], who measured FF for anterior, medial and posterior thigh muscle compartments. Between 30 and 70 years FF of the posterior compartment increased from 4.4 to 9.8% between youngest and oldest decades compared to an increase of 1.8–2.8% of the anterior and of 1.8–3.9% of the medial compartments. Our data of muscle tissue FF showed an increase from 4.1% at age 35 to 5.5% at age 65.
For the analysis of paraspinal muscles, we performed a manual segmentation of a single slice, only. Crawford et al. [45] reported FF and volume of the erector spinae for 20–60-year-old women and men. In agreement with our results there was no significant age-related change in erector volume and a significant increase in FF. Similar to our results, Dallaway et al. reported a decrease in muscle volume of the erector spinae but not of the psoas in older compared to young males. However, fat infiltration decreased in both muscles [46]. CT data from a large study in 516 Chinese women showed a quadratic increase of IMAT area and of % IMAT from age 20 to age 80 [29]. Of course, it is difficult to compare CT and MRI imaging results but our results showed very comparable age-related changes of IMAT and muscle volume.
From a clinical perspective our data represent an important contribution of imaging to identify and characterize both reversible and irreversible pathologic changes in muscle tissue that could contribute to tissue degeneration and widespread age-associated diseases such as sarcopenia [8, 47], osteoporosis and fragility fractures [48,49,50] and low back pain [51, 52]. Such findings could both be diagnostic and prognostic markers that influence prevention and treatment decisions. Myosteatosis has recently been recognized as an indicator that reflects metabolic, inflammatory and degenerative pathology and the crosstalk between them [53]. Although myosteatosis is discussed as a separate entity by several authors, it can propagate dysfunction of muscle, bone and tendons, just to name some relevant musculoskeletal tissues. Fatty infiltration of muscle tissue can be initiated by metabolic pathology such as obesity, insulin resistance and diabetes combined with disuse. Alternatively a chronic inflammatory microenvironment may trigger adipogenic and fibrogenic differentiation of muscle stem cells and may further propagate inflammatory myosteatosis. The dystopic and dysregulated adipose tissue secretes pro inflammatory adipokines and as such is part of a vicious cycle that further propagates “inflammaging” and tissue degeneration. Moreover, this microenvironment may also trigger fibrotic scarring as a result of altered tissue regeneration. Taken together the resulting cascades of degeneration promote.
In conclusion and in agreement with other publications in healthy men, thigh muscle volume decreased with age and fat fraction increased. In the spine, similar changes were observed for the erector spinae but not for the psoas. An adjustment of volumetric parameters by FL or MT volume is advisable when comparing age-dependent results in cross-sectional studies as adjusted parameters are more sensitive to muscle atrophy of the thigh. Advanced imaging methods to differentiate EMCL and IMCL effects should be developed.
References
Cruz-Jentoft AJ et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Goodpaster BH et al (2001) Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J Appl Physiol (1985) 90:2157–2165
Goodpaster BH et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Addison O et al (2014) Intermuscular fat: a review of the consequences and causes. Int J Endocrinol 2014:309570
Buch A et al (2016) Muscle function and fat content in relation to sarcopenia, obesity and frailty of old age—an overview. Exp Gerontol 76:25–32
Hilton TN et al (2008) Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 88:1336–1344
Visser M et al (2005) Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 60:324–333
Zamboni M, Gattazzo S, Rossi AP (2019) Myosteatosis: a relevant, yet poorly explored element of sarcopenia. Eur Geriatr Med 10:5–6
Baum T et al (2016) Association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. J Comput Assist Tomogr 40:447–451
Inhuber S et al (2019) Associations of thigh muscle fat infiltration with isometric strength measurements based on chemical shift encoding-based water-fat magnetic resonance imaging. Eur Radiol Exp 3:45
Karampinos DC et al (2012) Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging 35:899–907
Mercuri E et al (2004) Congenital form of spinal muscular atrophy predominantly affecting the lower limbs: a clinical and muscle MRI study. Neuromuscul Disord 14:125–129
Grimm A et al (2018) Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J Cachexia Sarcopenia Muscle 9:1093–1100
Grimm A et al (2019) A comparison between 6-point Dixon MRI and MR spectroscopy to quantify muscle fat in the thigh of subjects with sarcopenia. J Frailty Aging 8:21–26
Boesch C et al (1997) In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med 37:484–493
Chaudry O et al (2021) Segmentation of the fascia lata and reproducible quantification of intermuscular adipose tissue (IMAT) of the thigh. MAGMA 34:367–376
Benjamin M (2009) The fascia of the limbs and back–a review. J Anat 214:1–18
Buford TW et al (2012) Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol 47:38–44
Marcus RL et al (2012) Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res 2012:629637
Akima H et al (2015) Skeletal muscle size is a major predictor of intramuscular fat content regardless of age. Eur J Appl Physiol 115:1627–1635
Addison O et al (2014) Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging 18:532–538
Ghasemikaram M et al (2021) Effects of 16 months of high intensity resistance training on thigh muscle fat infiltration in elderly men with osteosarcopenia. Geroscience 43:607–617
Bruseghini P et al (2019) Effects of high-intensity interval training and isoinertial training on leg extensors muscle function, structure, and intermuscular adipose tissue in older adults. Front Physiol 10:1260
Farrow M et al (2021) The effect of ageing on skeletal muscle as assessed by quantitative MR imaging: an association with frailty and muscle strength. Aging Clin Exp Res 33:291–301
Hogrel JY et al (2015) NMR imaging estimates of muscle volume and intramuscular fat infiltration in the thigh: variations with muscle, gender, and age. Age (Dordrecht) 37:9798
Yoon MA et al (2018) Multiparametric MR imaging of age-related changes in healthy thigh muscles. Radiology 287:235–246
Delmonico MJ et al (2009) Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90:1579–1585
Akazawa N et al (2020) Quantitative features of intramuscular adipose tissue of the quadriceps and their association with gait independence in older inpatients: a cross-sectional study. Nutrition 71:110600
Peng X et al (2020) Age-related fatty infiltration of lumbar paraspinal muscles: a normative reference database study in 516 Chinese females. Quant Imaging Med Surg 10:1590–1601
Burian E et al (2020) Association of thigh and paraspinal muscle composition in young adults using chemical shift encoding-based water-fat MRI. Quant Imaging Med Surg 10:128–136
Zink-Ruckel C et al (2021) Once weekly whole-body electromyostimulation enhances muscle quality in men: data of the randomized controlled Franconian electromyostimulation and golf study. Front Physiol 12:700423
World Medical, A. (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Rossi A et al (2010) Quantification of intermuscular adipose tissue in the erector spinae muscle by MRI: agreement with histological evaluation. Obesity (Silver Spring) 18:2379–2384
Song MY et al (2004) Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 79:874–880
German’s health status and health-related body measurements reported by Federal Office of Statistics destatis.de (2017). https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Gesundheitszustand-Relevantes-Verhalten/Tabellen/koerpermasse-maenner.html
Correa-de-Araujo R et al (2020) Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the National Institute on Aging. Front Physiol 11:963
Ogon I et al (2020) Is the lipid content of the psoas major correlated with chronic low back pain and spinopelvic alignment? A magnetic resonance spectroscopic study. Asian Spine J 14:430–437
Uezumi A et al (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124:3654–3664
Parker E, Hamrick MW (2021) Role of fibro-adipogenic progenitor cells in muscle atrophy and musculoskeletal diseases. Curr Opin Pharmacol 58:1–7
Molina T, Fabre P, Dumont NA (2021) Fibro-adipogenic progenitors in skeletal muscle homeostasis, regeneration and diseases. Open Biol 11:210110
Tan Y et al (2021) Lipid droplets contribute myogenic differentiation in C2C12 by promoting the remodeling of the acstin-filament. Cell Death Dis 12:1102
Daemen S et al (2018) Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol Metab 17:71–81
Dube JJ et al (2008) Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. Am J Physiol Endocrinol Metab 294:E882–E888
Goodpaster BH et al (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86:5755–5761
Crawford RJ et al (2016) Age- and level-dependence of fatty infiltration in lumbar paravertebral muscles of healthy volunteers. AJNR Am J Neuroradiol 37:742–748
Dallaway A et al (2020) Age-related degeneration of lumbar muscle morphology in healthy younger versus older men. Aging Male 23:1583–1597
Ahn H et al (2021) Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: a new paradigm beyond sarcopenia. Ageing Res Rev 70:101398
Colon-Emeric C et al (2020) AGS and NIA bench-to bedside conference summary: osteoporosis and soft tissue (muscle and fat) disorders. J Am Geriatr Soc 68:31–38
Martel D et al (2020) Analysis of muscle, hip, and subcutaneous fat in osteoporosis patients with varying degrees of fracture risk using 3T chemical shift encoded MRI. Bone Rep 12:100259
Wong AK et al (2020) Bone marrow and muscle fat infiltration are correlated among postmenopausal women with osteoporosis: the AMBERS cohort study. J Bone Miner Res 35:516–527
Prasetyo M et al (2020) Computed tomography evaluation of fat infiltration ratio of the multifidus muscle in chronic low back pain patients. Eur J Radiol Open 7:100293
Sollmann N et al (2022) Paraspinal muscle in chronic low back pain: comparison between standard parameters and chemical shift encoding-based water-fat MRI. J Magn Reson Imaging. https://doi.org/10.1002/jmri.28145
Herrmann M et al (2020) Interactions between muscle and bone-where physics meets biology. Biomolecules 10:432. https://doi.org/10.3390/biom10030432
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any financial or non-financial interests that are directly or indirectly related to the work submitted for publication. Klaus Engelke is a part time employee of Clario, Inc.
Ethical approval
This was a retrospective analysis of data acquired for the Franconian EMS and Golf (FREMGO) study [31]. FREMGO had been approved by the Friedrich-Alexander University Erlangen-Nürnberg (FAU) Ethics Committee (number 377_19b) and registered under ClinicalTrials.gov: NCT04264416.
Statement of human and animal rights
The study fully complied with the Helsinki Declaration “Ethical Principles for Medical Research Involving Human Subjects” [32].
Informed consent
All study participants gave their written informed consent after having received detailed information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Engelke, K., Ghasemikaram, M., Chaudry, O. et al. The effect of ageing on fat infiltration of thigh and paraspinal muscles in men. Aging Clin Exp Res 34, 2089–2098 (2022). https://doi.org/10.1007/s40520-022-02149-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-022-02149-1