Abstract
Purpose
The purpose of this study was to evaluate the association between clinically possible rapid eye movement (REM) sleep behavioral disorder (pRBD) and orthostatic hypotension (OH) in PD patients, as well as to explore the mechanisms underlying the association.
Methods
PD patients (n = 116) were assigned to a group with OH (PD-OH) or without OH (PD-NOH). General demographic and clinical data were collected. A series of scales were used to assess the clinical symptoms in the two groups.
Results
A total of 27 patients (23.3%) had OH. The PD-OH group showed significantly higher H-Y staging score and significantly higher frequencies of pRBD, anxiety, depression, and cognitive impairment than the PD-NOH group. Binary logistic regression analysis identified the following factors as independently associated with PD-OH: H-Y staging [odds ratio (OR) 2.565, 95% confidence interval (CI) 1.160–5.673; P = 0.020], RBD (OR 7.680, 95% CI 1.944–30.346; P = 0.004), UPDRS II (OR 1.021, 95% CI 0.980–1.063; P = 0.020), depression (OR 7.601, 95% CI 1.492–38.718; P = 0.015), and cognitive impairment (OR 0.824, 95% CI 0.696–0.976; P = 0.025).
Conclusions
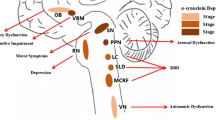
Our results suggest that pRBD is an independent risk factor for OH in patients with PD. We speculate that there may be a close relationship between RBD and OH, which requires attention. Early diagnosis of RBD may help predict the appearance of OH in PD patients.

Similar content being viewed by others
References
Coon EA, Cutsforth-Gregory JK, Benarroch EE (2018) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33:349–358
Fereshtehnejad SM, Lokk J (2014) Orthostatic hypotension in patients with Parkinson’s disease and atypical parkinsonism. Parkinsons Dis 2014:475854
Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223
St Louis EK, Boeve AR, Boeve BF (2017) REM sleep behavior disorder in Parkinson’s disease and other synucleinopathies. Mov Disord 32:645–658
Högl B, Stefani A, Videnovic A (2018) Idiopathic REM sleep behaviour disorder and neurodegeneration—an update. Nat Rev Neurol 14:40–55
Ng M, Pavlova M (2013) Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages. Epilepsy Res Treat 2013:932790
Jain S, Goldstein DS (2012) Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 46:572–580
Arnaldi D, De Carli F, Famà F et al (2017) Prediction of cognitive worsening in de novo Parkinson’s disease: clinical use of biomarkers. Mov Disord 32:1738–1747
Joong-Seok K, Hyung-Eun P, Yoon-Sang O et al (2016) Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J Neurol Sci 362:59–63
Fereshtehnejad S-M, Romenets SR, Anang JBM et al (2015) New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 72:863–873
Pilotto A, Romagnolo A, Tuazon Jasmine A et al (2019) Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in α-synucleinopathies. J Neurol Neurosurg Psychiatry 90:1257–1263
Goldman JG, Holden SK, Litvan I et al (2018) Evolution of diagnostic criteria and assessments for Parkinson’s disease mild cognitive impairment. Mov Disord 33:503–510
Pilotto A, Premi E, Paola Caminiti S et al (2018) Single-subject SPM FDG-PET patterns predict risk of dementia progression in Parkinson disease. Neurology 90:e1029–e1037
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson‘s disease. Mov Disord 30:1591–1601
Freeman R, Wieling W, Axelrod FB et al (2011) Consensus statement on the defifinition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 21:69–72
Choe YM, Lee B, Choi I-G et al (2020) MMSE subscale scores as useful predictors of AD conversion in mild cognitive impairment. Neuropsychiatr Dis Treat 16:1767–1775
Hussain MW, Camicioli R (2018) Nonmotor symptoms of Parkinson’s disease as predictors of dementia. Can J Neurol Sci 45:97–99
Tanaka R, Shimo Y, Yamashiro K et al (2018) Association between abnormal nocturnal blood pressure profile and dementia in Parkinson’s disease. Parkinsonism Relat Disord 46:24–29
Li L, Guo P, Ding D et al (2019) Parkinson’s disease with orthostatic hypotension: analyses of clinical characteristics and influencing factors. Neurol Res 41:734–741
Bonuccelli U, Lucetti C, Del Dotto P et al (2003) Orthostatic hypotension in de novo Parkinson disease. Arch Neurol 60:1400–1404
Lee HM, Koh SB (2015) Many faces of Parkinson’s disease: non-motor symptoms of Parkinson’s disease. J Mov Disord 8:92–97
Hohler AD, Zuzuárregui JR, Katz DI et al (2012) Differences in motor and cognitive function in patients with Parkinson’s disease with and without orthostatic hypotension. Int J Neurosci 122:233–236
Rose KM, Couper D, Eigenbrodt ML et al (2010) Orthostatic hypotension and cognitive function: the atherosclerosis risk in communities study. Neuroepidemiology 34:1–7
Senard JM, Valet P, Durrieu G et al (1990) Adrenergic supersensitivity in Parkinsonians with orthostatic hypotension. Eur J Clin Invest 20:613–619
Choi MH, Yoon JH, Yong SW (2017) Cardiac sympathetic denervation and dementia in de novo Parkinson’s disease: a 7-year follow-up study. J Neurol Sci 381:291–295
Haubrich C, Pies K, Dafotakis M et al (2010) Transcranial doppler monitoring in Parkinson’s disease: cerebrovascular compensation of orthostatic hypotension. Ultrasound Med Biol 36:1581–1587
Centi J, Freeman R, Gibbons CH et al (2017) Effects of orthostatic hypotension on cognition in Parkinson disease. Neurology 88:17–24
Oh YS, Kim JS, Lee KS (2013) Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J Mov Disord 6:23–27
Perlmuter LC, Sarda G, Casavant V et al (2013) A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am J Ther 20:279–291
Broen MP, Narayen NE, Kuijf ML et al (2016) Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. J Mov Disord Soc 31:1125–1133
Reijnders JS, Ehrt U, Weber WE et al (2008) A systematic review of prevalence studies of depression in Parkinson’s disease. J Mov Disord Soc 23:183–189
Bayulkem K, Lopez G (2010) Nonmotor flfluctuations in Parkinson’s disease: clinical spectrum and classifification. J Neurol Sci 289:89–92
Hu X, Song X, Yuan Y et al (2015) A bnormal functional connectivity of the amygdala is associated with depression in Parkinson’sdisease. Mov Disord 30:238–244
Weintraub D, Newberg AB, Cary MS et al (2005) Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med 46:227–232
Rudorfer MV, Ross R, Linnoila M et al (1985) Exaggerated orthostatic responsivity of plasma norepinephrine in depression. Arch Gen Psychiatry 42:1186–1192
Voss A, Schulz BV, Bar KJ (2006) Linear and nonlinear methods for analyses of cardiovascular variability in bipolar disorders. Bipolar Disord 8:441–452
Acknowledgements
We thank the Parkinson’s disease patients for their participation in our study.
Funding
This work was funded by the National Natural Science Foundation of China (grant number 81960242), Yunnan Applied Basic Research Project (grant numbers 2019FE001-048 and 202001AT070001), Yunnan Provincial Health Science and Technology Project (grant number 2018NS0102), The Applied Basic Research of The Diagnosis and Treatment Center of Neurological Diseases in Yunnan Province (grant number ZX2019-03-05) and the 100 Young and Middle-aged Academic and Technical Scholars at Kunming Medical University (grant number 60118260105).
Author information
Authors and Affiliations
Contributions
KFY contributed to the acquisition of the data, statistical analysis, and interpretation of the data, and drafted the manuscript. ZX and XLY contributed to the study concept and design, acquisition of the data, statistical analysis, and critical revision of the manuscript for important intellectual content. YYZ, WFY, CBZ, LY, YFZ, BL and HR contributed to the acquisition of the data and clinical assessment.
Corresponding author
Ethics declarations
Conflicts of interest
All authors state that they have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was obtained from all patients and their families for their anonymized clinical data to be published for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, K., Zhou, C., Zhu, Y. et al. REM sleep behavioral disorder may be an independent risk factor for orthostatic hypotension in Parkinson’s disease. Aging Clin Exp Res 34, 159–166 (2022). https://doi.org/10.1007/s40520-021-01887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-01887-y