Abstract
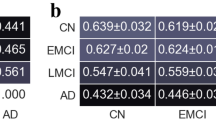
The disease roots of Alzheimer’s disease (AD) are unknown. Functional connection (FC) methodology based on functional MRI data is an effective lever to investigate macroscopic neural activity patterns. However, regional properties of brain architecture have been less investigated by special markers of graph indexes in general mental disorders. In terms of the set of the abnormal edges in the FCs matrix, this paper introduces the strength index (S-scores) of region centrality on the principle of holism. Then, the important process is to investigate the S-scores of regions and subsystems in 36 healthy controls, 38 mild cognitive impairment (MCI) patients and 34 AD patients. At the edge level, abnormal FCs is numerically increasing progressively from MCI to AD brains. At the region level, the CUN.L, PAL.R, THA.L, and TPOsup.R regions are highlighted with abnormal S-scores in MCI patients. By comparison, more regions are abnormal in AD patients, which are PreCG.L, INS.R, DCG.L, AMYG.R, IOG.R, FFG.L, PoCG.L, PCUN.R, TPOsup.L, MTG.L, and TPOmid.L. Importantly, the regions in DMN have abnormal S-scores in AD groups. At the module level, the S-scores of frontal, parietal, occipital lobe, and cerebellum are found in MCI and AD patients. Meanwhile, the abnormal lateralization is inferred because of the S-scores of left and top hemisphere in the AD group. Though this is strictly a contrastive study, the S-score may be a meaningful imaging marker for excavating AD psychopathology.




Similar content being viewed by others
References
Blennow K, de Leon MJ, Zetterberg H (2006) Alzheimer’s disease. Lancet 368:387–403
Dubois B, Feldman HH, Jacova C et al (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–11127
Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362:329–344
Gauthier S, Reisberg B, Zaudig M et al (2006) (2006) Mild cognitive impairment. Lancet 367:1262–1270
Ballard C, Gauthier S, Corbett A et al (2011) Alzheimer’s disease. Lancet 377:1019–1031
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:263–269
Brunden KR, Trojanowski JQ, Lee VM (2009) Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov 8:783–793
Bashan A, Bartsch RP, Kantelhardt JW et al (2012) Network physiology reveals relations between network topology and physiological function. Nat Commun 3:702
Wang XJ, Krystal JH (2014) Computational psychiatry. Neuron 84:638–654
Dubois B, Feldman HH, Jacova C et al (2014) Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 13:614–629
Bassett DS, Sporns O (2017) Network neuroscience. Nat Neurosci 20:353–363
Bassett DS, Zurn P, Gold JI (2018) On the nature and use of models in network neuroscience. Nat Rev Neurosci 19:566–578
Mattson MP (2004) Pathways towards and away from Alzheimer's disease. Nature 430:631–639
Liu CC, Liu CC, Kanekiyo T et al (2013) Apolipoprotein E and Alzheimer’s disease: Risk, mechanisms and therapy. Nat Rev Neurol 9:106–118
Agosta F, Weiler M, Filippi M (2015) Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes. CNS Neurosci Ther 21:754–767
Brettschneider J, Del Tredici K, Lee VM et al (2015) Spreading of pathology in neurodegenerative diseases: a focus on human studies. Nat Rev Neurosci 16:109–120
D’Esposito M, Deouell LY, Gazzaley A (2003) Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci 4:863–872
Power JD, Schlaggar BL, Lessov-Schlaggar CN et al (2013) Evidence for hubs in human functional brain networks. Neuron 79:798–813
Greicius MD, Srivastava G, Reiss AL et al (2004) Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci 101:4637–4642
Pakkenberg B, Pelvig D, Marner L et al (2003) Aging and the human neocortex. Exp Gerontol 38:95–99
Karas GB, Burton EJ, Rombouts SA et al (2003) A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. NeuroImage 18:895–907
Henneman WJ, Sluimer JD, Barnes J et al (2009) Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology 72:999–1007
Grady C (2012) The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505
Lee MH, Smyser CD, Shimony JS (2013) Resting-state fMRI: a review of methods and clinical applications. Am J Neuroradiol 34:1866–1872
Pievani M, Filippini N, van den Heuvel MP et al (2014) Brain connectivity in neurodegenerative diseases–-from phenotype to proteinopathy. Nat Rev Neurol 10:620–633
Hohenfeld C, Werner CJ, Reetz K (2018) Resting-state connectivity in neurodegenerative disorders: is there potential for an imaging biomarker? Neuroimage Clin 18:849–870
Chandra A, Dervenoulas G, Politis M et al (2019) Magnetic resonance imaging in Alzheimer’s disease and mild cognitive impairment. J Neurol 266:1293–1302
Laakso MP, Soininen H, Partanen K et al (1995) Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: correlation with memory functions. J Neural Transm 9:73–86
Lerch JP, Pruessner JC, Zijdenbos A et al (2005) Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex 15:995–1001
Teipel SJ, Pruessner JC, Faltraco F et al (2006) Comprehensive dissection of the medial temporal lobe in AD: Measurement of hippocampus, amygdala, entorhinal, perirhinal and parahippocampal cortices using MRI. J Neurol 253:794–800
Small SA, Perera GM, DeLaPaz R et al (1999) Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol 45:466–472
Celone KA, Calhoun VD, Dickerson BC et al (2006) Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci 26:10222–10231
Sorg C, Riedl V, Mühlau M et al (2007) Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci 104:18760–18765
Biswal BB, Mennes M, Zuo XN et al (2010) Toward discovery science of human brain function. Proc Natl Acad Sci 107:4734–4739
Cole DM, Smith SM, Beckmann CF (2010) Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Front Syst Neurosci 4:8
van den Heuvel MP, Hulshoff Pol HE (2010) Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharm 20:519–534
Liang X, Zou Q, He Y et al (2013) Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci 110:1929–1934
Aiello M, Salvatore E, Cachia A et al (2015) Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage 113:111–121
Fornito A, Zalesky A, Breakspear M (2015) The connectomics of brain disorders. Nat Rev Neurosci 16:159–172
Sheline YI, Raichle ME (2013) Resting state functional connectivity in preclinical Alzheimer’s disease. Biol Psychiatry 74:340–347
He Y, Evans A (2010) Graph theoretical modeling of brain connectivity. Curr Opin Neurol 23:341–350
Binnewijzend MA, Schoonheim MM, Sanz-Arigita E et al (2012) Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 33:2018–2028
Zarei M, Beckmann CF, Binnewijzend MA et al (2013) Functional segmentation of the hippocampus in the healthy human brain and in Alzheimer’s disease. Neuroimage 66:28–35
Zhang HY, Wang SJ, Xing J et al (2009) Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behav Brain Res 197:103–108
Pasquini L, Scherr M, Tahmasian M et al (2015) Link between hippocampus’ raised local and eased global intrinsic connectivity in AD. Alzheimers Dement 11:475–484
Badhwar A, Tam A, Dansereau C et al (2017) Resting-state network dysfunction in Alzheimer's disease: a systematic review and meta-analysis. Alzheimers Dement 18:73–85
Schouten TM, Koini M, de Vos F et al (2016) Combining anatomical, diffusion, and resting state functional magnetic resonance imaging for individual classification of mild and moderate Alzheimer’s disease. Neuroimage Clin 11:46–51
de Vos F, Koini M, Schouten TM et al (2018) A comprehensive analysis of resting state fMRI measures to classify individual patients with Alzheimer’s disease. Neuroimage 167:62–72
Liu Y, Liang M, Zhou Y et al (2008) Disrupted small-world networks in schizophrenia. Brain 131:945–961
Wang J, Zuo X, Dai Z et al (2013) Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry 73:472–481
Zuo XN, Di Martino A, Kelly C et al (2010) The oscillating brain: complex and reliable. Neuroimage 49:1432–1445
Leopold DA, Murayama Y, Logothetis NK (2003) Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex 13:422–433
Clare S, Bridge H (2005) Methodological issues relating to in vivo cortical myelography using MRI. Hum Brain Mapp 26:240–250
Devlin JT, Poldrack RA (2007) In praise of tedious anatomy. Neuroimage 37:1033–1041
Yeo BT, Krienen FM, Sepulcre J et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165
Hagmann P, Cammoun L, Gigandet X et al (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159
Dennis EL, Thompson PM (2014) Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev 24:49–62
Dai ZJ, Yan CG, Li KC et al (2015) Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb Cortex 25:3723–3742
Lord LD, Stevner AB, Deco G et al (2017) Understanding principles of integration and segregation using whole-brain computational connectomics: implications for neuropsychiatric disorders. Philos Trans A 375:1–21
Stam C, Jones B, Nolte G et al (2007) Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex 17:92–99
Liu Y, Yu C, Zhang X et al (2014) Impaired long distance functional connectivity and weighted network architecture in Alzheimer’s disease. Cereb Cortex 24:1422–1436
Baron JC, Chételat G, Desgranges B et al (2001) In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. NeuroImage 14:298–309
De Jong LW, Der Van HK, Veer IM et al (2008) Strongly reduced volumes of putamen and thalamus in Alzheimer's disease: an MRI study. Brain 131:3277–3285
Rombouts SA, Barkhof F, Witter MP et al (2000) Unbiased whole-brain analysis of gray matter loss in Alzheimer’s disease. Neurosci Lett 285:231–233
Dickerson BC, Sperling RA (2008) Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46:1624–1635
Sperling RA, Bates JF, Chua EF et al (2003) fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:44–50
Pariente J, Cole S, Henson R et al (2005) Alzheimer’s patients engage an alternative network during a memory task. Ann Neurol 58:870–879
Leal SL, Landau SM, Bell RK et al (2017) Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife 6:e22978
Allen G, Barnard H, McColl R et al (2007) Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol 64:1482–1487
Wang L, Zang Y, He Y et al (2006) Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. NeuroImage 31:469–504
Ferrarini L, Palm WM, Olofsen H et al (2006) Shape differences of the brain ventricles in Alzheimer’s disease. Neuroimage 32:1060–1069
Lin F, Ren P, Lo RY et al (2017) Insula and inferior frontal gyrus activities protect memory performance against Alzheimer's disease pathology in old age. J Alzheimers Dis 55:669–678
Yao HX, Liu Y, Zhou B et al (2013) Decreased functional connectivity of the amygdala in Alzheimer’s disease revealed by resting-state fMRI. Eur J Radiol 82:1531–1538
Buckner RL, Snyder AZ, Shannon BJ et al (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717
Zhang ZQ, Liu Y, Jiang TZ et al (2012) Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage 59:1429–1440
Rombouts SA, Barkhof F, Goekoop R et al (2005) Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum Brain Mapp 26:231–239
Duara R, Loewenstein DA, Potter E et al (2008) Medial temporal lobe atrophy on MRI scans and the diagnosis of Alzheimer disease. Neurology 71:1986–1992
Machulda MM, Ward HA, Borowski B et al (2003) Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology 61:500–506
Das SR, Pluta J, Mancuso L et al (2015) Anterior and posterior MTL networks in aging and MCI. Neurobiol Aging 36:S141–S150
Cha J, Hang JJ, Kim HJ et al (2013) Functional alteration patterns of default mode networks: comparisons of normal aging, amnestic mild cognitive impairment and Alzheimer's disease. Eur J Neurosci 37:1916–1924
Weiler M, Agosta F, Canu E et al (2015) Following the spreading of brain structural changes in Alzheimer’s disease: a longitudinal, multimodal MRI study. J Alzheimers Dis 47:995–1007
Rombouts SA, Barkhof F, Van Meel CS et al (2002) Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 73:665–671
Hao J, Li K, Li K et al (2005) Visual attention deficits in Alzheimer’s disease: an fMRI study. Neurosci Lett 385:18–23
Agosta F, Pievani M, Geroldi C et al (2012) Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol Aging 33:1564–1578
Zamboni G, Wilcock GK, Douaud G et al (2013) Resting functional connectivity reveals residual functional activity in Alzheimer’s disease. Biol Psychiatry 74:375–383
Sui X, Zhu M, Cui Y et al (2015) Functional connectivity hubs could serve as a potential biomarker in Alzheimer’s disease: a reproducible study. Curr Alzheimer Res 12:974–983
Jacobs HIL, Hopkins DA, Mayrhofer HC et al (2018) The cerebellum in Alzheimer's disease: evaluating its role in cognitive decline. Brain 141:37–47
Wegiel J, Wisniewski HM, Dziewiatkowski J et al (1999) Cerebellar atrophy in Alzheimer’s disease—clinicopathological correlations. Brain Res 818:41–50
Tabatabaei-Jafari H, Walsh E, Shaw ME et al (2017) The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 38:3141–3150
Balsters JH, Laird AR, Fox PT et al (2014) Bridging the gap between functional and anatomical features of cortico-cerebellar circuits using meta-analytic connectivity modeling. Hum Brain Mapp 35:3152–3169
Kim JH, Lee JW, Kim GH et al (2012) Cortical asymmetries in normal, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging 33:1959–1966
Long X, Zhang L, Liao W et al (2013) Distinct laterality alterations distinguish mild cognitive impairment and Alzheimer’s disease from healthy aging: statistical parametric mapping with high resolution MRI. Hum Brain Mapp 34:3400–3410
Barnes J, Scahill RI, Schott JM et al (2005) Does Alzheimer's disease affect hippocampal asymmetry? Evidence from a cross-sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord 19:338–344
Filippi M, Basaia S, Canu E et al (2017) Brain network connectivity differs in early-onset neurodegenerative dementia. Neurology 89:1764–1772
Banks SJ, Zhuang X, Bayram E et al (2018) Default mode network lateralization and memory in healthy aging and Alzheimer’s disease. J Alzheimers Dis 66:1223–1234
Yang C, Zhong S, Zhou X et al (2017) The abnormality of topological asymmetry between hemispheric brain white matter networks in Alzheimer’s disease and mild cognitive impairment. Front Aging Neurosci 9:261
Borst G, Thompson WL, Kosslyn SM (2011) Understanding the dorsal and ventral systems of the human cerebral cortex: beyond dichotomies. Am Psychol 66:624–632
Vossel S, Geng JJ, Fink GR (2014) Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. Neuroscientist 20:150–159
Andrews-Hanna JR, Reidler JS, Sepulcre J et al (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65:550–562
Adriaanse SM, Binnewijzend MA, Ossenkoppele R et al (2014) Widespread disruption of functional brain organization in early-onset Alzheimer’s disease. PLoS ONE 9:e102995
Thompson PM, Hayashi KM, de Zubicaray G et al (2003) Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci 23:994–1005
Lustig C, Snyder AZ, Bhakta M et al (2003) Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci 100:14504–14509
Greicius MD, Supekar K, Menon V et al (2009) Resting-state functional connectivity reflects structural connectivity in the default-mode network. Cereb Cortex 19:72–78
Funding
The author did not receive any funding or support for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Statement of human and animal rights
All procedures performed in the study were in accordance with the ethical standards of the institution and the national research committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
About this article
Cite this article
Chen, B. Abnormal cortical regions and subsystems in whole brain functional connectivity of mild cognitive impairment and Alzheimer’s disease: a preliminary study. Aging Clin Exp Res 33, 367–381 (2021). https://doi.org/10.1007/s40520-020-01539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01539-7