Abstract
Background
Changes in well-being of patients with multiple myeloma (MM) before and after diagnosis have not been quantified.
Aims
Explore the use of secondary data to examine the changes in the well-being of older patients with MM.
Methods
We used the Health and Retirement Study (HRS), linked to Medicare claims to identify older MM patients. We compared patient-reported measures (PRM), including physical impairment, sensory impairment, and patient experience (significant pain, self-rated health, depression) in the interviews before and after MM diagnosis using McNemar’s test. We propensity-matched each MM patient to five HRS participants without MM diagnosis based on baseline characteristics. We compared the change in PRM between the MM patients and their matches.
Results
We identified 92 HRS patients with MM diagnosis (mean age = 74.6, SD = 8.4). Among the surviving patients, there was a decline in well-being across most measures, including ADL difficulty (23% to 40%, p value = 0.016), poor or fair self-rated health (38% to 61%, p value = 0.004), and depression (15% to 30%, p value = 0.021). Surviving patients reported worse health than participants without MM across most measures, including ADL difficulty (40% vs. 27%, p value = 0.04), significant pain (38% vs. 22%, p value = 0.01), and depression (29% vs. 11%, p value = 0.003).
Discussion
Secondary data were used to identify patients with MM diagnosis, and examine changes across multiple measures of well-being. MM diagnosis negatively affects several aspects of patients’ well-being, and these declines are larger than those experienced by similar participants without MM.
Conclusion
The results of this study are valuable addition to understanding the experience of patients with MM, despite several data limitations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma is a rare disease, affecting approximately 7 out of 100,000 persons in the United States each year. Multiple myeloma disproportionately affects older patients, with a median age at diagnosis being over 70 years old [1]. While new treatments are increasing the survival time [2], we know less about the quality of life of older persons who are more susceptible to treatment related adverse events. Older patients are concerned not only with survival time, but also with symptom management, maintaining independence, and how their well-being will be affected by the disease and treatments [3]. Previous studies have shown that patients with multiple myeloma experience higher symptom burden and lower quality of life compared to the general population [4, 5], but these studies did not focused on older patients specifically. Overall, there is a consensus that older patients with multiple myeloma could benefit from a geriatric assessment before and during treatment [3, 6, 7]. However, to our knowledge, there have been no studies that formally quantify patient-reported measures in older multiple myeloma patients before and after the diagnosis, or studies comparing the changes in well-being to older adults experiencing the normal ageing process.
As with any rare disease, it can be difficult to recruit large numbers of multiple myeloma patients for research studies due to the low prevalence of the disease, which makes recruiting large numbers of patients resource intensive. One potential source of data for studies of rare diseases is secondary data, including large observational studies and administrative data. In this study we explore studying the well-being of older multiple myeloma patients using Medicare claims data, and Health and Retirement Study (HRS), an ongoing longitudinal study started in 1992. The use of this longitudinal cohort has several advantages. First, due to length of the study period, enough cases accrue over time, creating a feasible study sample. Second, HRS is a detailed study with data on a variety of health status measures that are of interest for older patients. Third, since HRS is a longitudinal study, each surviving participant is interviewed every 2 years, meaning that the measures of interest are observed before and after the diagnosis. Fourth, we can compare changes in the health status measures in HRS participants without multiple myeloma, for whom we have the same health status measures over the same period of time.
The use of HRS data that were not collected for the purpose of learning about well-being of multiple myeloma patients imposes limits on questions that can be answered. For example, since we do not have the information on what treatment the patients receive, we can not determine if well-being changes are due to the disease or its treatment. In addition, because the HRS interviews are biennial, the time between multiple myeloma diagnosis and report of health status measures varies between 0 days and 2 years. However, HRS data also make it possible to address issues that would be difficult in a primary data collection study. It makes it possible to collect data on prediagnosis health status rather than retrospective reports. In addition, we can compare changes in health to population-representative subjects without multiple myeloma.
We conducted this study examining changes before and after multiple myeloma diagnosis with three specific goals: (1) assessing feasibility of using claims data to identify and study patients with multiple myeloma; (2) assessing the change in patient-reported measures before and after diagnosis; (3) comparing the change in patient-reported measures to a comparable sample of individuals without multiple myeloma.
Methods
Participants
The Health and Retirement Study (HRS) was designed to examine changes in health and wealth as people age [8]. It is an ongoing nationally representative longitudinal study of participants age 50 and older. The study started in 1992 and follow up surveys are administered every 2 years. If a participant is not able to complete an interview, the interview is conducted with a proxy respondent.
We examined participants who had a diagnosis of multiple myeloma while enrolled in the HRS, ascertained by linking the HRS survey data to Medicare claims. A participant was identified as having a diagnosis of multiple myeloma if he or she had two or more Medicare claims with ICD9 code 203.0. While we required two claims to confirm the diagnosis of multiple myeloma, the date of diagnosis was set to the date of first claim.
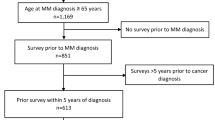
Out of 28,927 HRS participants age 65 or older at any point between 1992 and 2015, 26,044 (90.0%) agreed to have their HRS surveys linked to the Medicare claims. We identified 126 participants who had two or more claims with multiple myeloma. Since we wanted to ensure that the first claim observed in our files was indeed the first diagnosis of multiple myeloma, we excluded 21 (16.7%) participants if they were not enrolled continuously in fee-for-service Medicare for 6 months prior to the first observed claim with multiple myeloma diagnosis. Of the remaining 105 multiple myeloma patients, 13 (12.4%) did not complete a HRS interview within 3 years before a multiple myeloma diagnosis and were, therefore, excluded. This resulted in a sample of 92 multiple myeloma patients included in the study.
Measures
All baseline characteristics of study participants were taken from the last HRS interview before a diagnosis of multiple myeloma. Those included participant characteristics (age, gender, race, marital status, education, wealth, comorbidities, and health behaviors), and patient-reported measures of interest.
We considered nine patient-reported measures that cover a range of well-being for multiple myeloma patients, and are part of geriatric assessment of older patients [9, 10]. The included measures cover the domains that matter most to patients: functioning, sensory impairment, and general well-being. While the nine measures do not constitute together a validated questionnaire, each one of the measures has been validated or used in prior research of patients’ well-being. We include below the exact text of the survey question for each measure, and the justification for the new coding, when the questions were not used in the original form. Additionally, HRS website includes detailed documentation guides for all the measures used in this study [8]. Four measures describe limitations in physical functioning: difficulty in any Activities of Daily Living (ADL), difficulty in any Instrumental Activities of Daily Living (IADL), difficulty walking several blocks, and difficulty climbing one flight of stairs. ADL and IADL measures are standardized measures of assessing function in older adults in both clinical and research setting [11, 12]. Difficulty in any functional measure was assessed in the HRS by asking, “Because of a health or memory problem do you have any difficulty with [activity]?”. There were six ADLs (bathing, eating, walking across the room, transferring to and from bed, using toilet, and dressing), and five IADLs (using a phone, preparing hot meals, grocery shopping, managing financing, managing medications) included in the assessment. Next, two sensory impairment measures were included: vision and hearing impairment. Each of the two sensory impairments were assessed by asking, “Is your [hearing/eyesight] excellent, very good, good, fair, or poor using a [hearing aid/glasses or corrective lenses] as usual?”. Finally, three additional measures of general well-being were considered: experience of significant pain, self-rated health, and self-reported symptoms of depression. The presence of significant pain was determined using two questions. First, subjects were asked, “Are you often troubled with pain?”. Subjects who responded “Yes” were then asked, “How bad is the pain most of the time: mild, moderate or severe?” Subjects who responded “moderate” or “severe” were classified as experiencing significant pain. This classification of significant pain has been applied in previous studies [13, 14], because it reflects the American Geriatrics Society Guidelines for the Pharmacologic Management of Persistent Pain in Older Adults and the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, which both recommend that moderate or severe pain should prompt a clinical response [15, 16]. The SUPPORT study (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) also used this classification to categorize reports of pain at the end of life [17]. Symptoms of depression were measured using the eight-item Center for Epidemiologic Studies Depression Scale [18, 19]. Participants were classified as having symptoms consistent with the diagnosis of depression if they reported more than three symptoms.
The same nine patient-reported measures were also assessed at the first HRS interview after a diagnosis of multiple myeloma.
Statistical analysis
We started with describing the multiple myeloma patients in terms of demographics, socioeconomic status and health status. Next, we assessed the frequency of each patient-reported measure before a multiple myeloma diagnosis. Depression was not assessed for participants with proxy respondents; therefore, 10 patients were not included in the analysis of frequency and changes in depression. Questions about pain levels was not asked in early HRS interviews (1992–1993), so 5 patients were not included in the analyses invlolving significant pain. Analyses were conducted independently for each variable to prevent missing values from one variable affecting the sample size in the analysis of other variables.
Next, we compared the frequency of adverse health on the patient-reported measures before and after a multiple myeloma diagnosis. Only patients that completed the interview after their multiple myeloma diagnosis were included in this analysis, as the goal of this study was to describe well-being and changes in well-being of the patients who survive the initial period after multiple myeloma diagnosis. Patients who died within 2 years of multiple myeloma diagnosis, but completed an HRS interview between their diagnosis and death, were included in the study. The comparison of patients before and after multiple myeloma diagnosis was done using McNemar’s test.
Finally, we compared the change in frequency of adverse health on the patient-reported measures between HRS patients with a diagnosis of multiple myeloma to those without the diagnosis over the same time period, i.e., selecting subjects interviewed around the same time. To perform this comparison, we matched each patient with multiple myeloma to five individuals without multiple myeloma. The matching was performed using a mixture of propensity score matching and exact matching. Patients were matched on propensity score for being diagnosed with multiple myeloma, calculated using age, gender, race, marital status, education, wealth, number of comorbidities, and smoking status. Additionally, patients were matched on exact year of interview before and after a diagnosis of multiple myeloma, and on exact status of patient-reported measure for the multiple myeloma patient before the diagnosis. The matching was done separately for each patient-reported measure. This analysis was again limited only those patients who completed the HRS interview after a diagnosis of multiple myeloma, as our goal was to describe the experience among surviving patients.
Statistical analyses were performed using SAS 9.4 [20] and R 3.4 [21, 22] software. This study has been approved by the Center for Disease Control’s Institutional Review Board (CDC).
Results
Table 1 describes the prediagnosis baseline characteristics of the multiple myeloma patients included in this study. The mean age of 92 patients was 74.6 (SD = 8.4), and 47% were women. Forty-three percent of patients had 3 or more chronic conditions before diagnosis. The most common ADL difficulties were getting dressed (13%) and bathing (13%). The most common IADL difficulties shopping for groceries (17%) and preparing hot meals (17%). Eighteen patients (20%) died within 1 year, and 38 (41%) died within 2 years of multiple myeloma diagnosis. This mortality rate is comparable to mortality rates reported in other long-term studies of older adults [2, 23]. Of the 92 patients included in the study, 66 (72%) completed HRS interview after multiple myeloma diagnosis. Most subjects not completing an interview died before there scheduled interview. The median time between interview before multiple myeloma diagnosis and the diagnosis was 13.0 months (IQR 6.3–19.1), and the median time between the diagnosis and the first interview after the diagnosis was 12.6 months (6.0–18.0) (Table 1).
About a quarter of all multiple myeloma patients reported difficulty with at least one ADL (n = 22, 24%) before the diagnosis (Table 2). Twenty-nine patients (32%) reported difficulty with at least one IADL, 34 patients (37%) reported difficulty with walking several blocks, and 25 patients (27%) reported difficulty with climbing one flight of stairs. Vision impairment was reported by 29 patients (32%), and hearing impairment was reported by 20 patients (22%) before multiple myeloma diagnosis. About a quarter of the patients experienced significant pain (n = 20, 23%), and about a fifth reported symptoms of depression (n = 15, 19%). The most common impairment before multiple myeloma diagnosis was poor or fair self-reported health (n = 37, 40%).
Impairments in patient-reported measures in surviving multiple myeloma patients increased significantly after the diagnosis (Table 3). Patients reported higher rates of difficulty in three of the four physical measures, including increases in ADL difficulty (23% to 40%, p = 0.02), difficulty walking several blocks (30% to 60%, p < 0.001), and difficulty climbing one flight of stairs (25% to 47%, p = 0.003). There was no statistically significant change in IADL difficulty (30% to 41%, p = 0.13) The rate of depression symptoms doubled after the diagnosis (15% to 30%, p = 0.02), and more patients reported poor or fair self-rated health (38% to 61%, p = 0.004). There was no significant increase in patients experiencing significant pain (27% to 38%, p = 0.09). There was no change at all in the rates of vision impairment (30% to 30%, p = 1), but patients reported significant increase in hearing impairment (20% to 33%, p = 0.04).
Matched HRS participants without a multiple myeloma diagnosis reported lower rates of some patient-reported measures in the interview after the multiple myeloma diagnosis of the patients (Table 4), even though they were matched to have the same level of impairment before the diagnosis. Participants without multiple myeloma reported lower rates in ADL difficulty (40% vs. 27%, p = 0.04), difficulty walking several blocks (60% vs. 37%, p < 0.001), and difficulty climbing one flight of stairs (47% vs. 29%, p = 0.005). There were no statistically significant differences in IADL difficulty (41% vs. 32%, p = 0.21), vision impairment (30% vs. 26%, p = 0.52), or hearing impairment (33% vs. 29%, p = 0.44). Multiple myeloma patients reported higher rates of poor or fair self-rated health (61% vs. 39%, p = 0.001), depression symptoms (29% vs. 11%, p = 0.003), and significant pain (38% vs. 22%, p = 0.01).
Discussion
Using Medicare claims data linked to 26,044 HRS subjects, we identified 105 patients with multiple myeloma, 92 of which completed HRS interviews within 3 years of diagnosis. Our study showed that a multiple myeloma diagnosis negatively affects several aspects of patients’ well-being. Decline in several, but not all, patient-reported measures was significantly larger among surviving multiple myeloma patients than the changes among similar participants without multiple myeloma diagnosis. These findings have a number of limitations imposed by the nature of the HRS data, yet also provide new insights beyond what is possible with primary data collection.
Health and Retirement Study was not collected for the purpose of learning about multiple myeloma, yet the design and richness of HRS study makes it possible to describe important aspects of the patient experience. While the multiple myeloma sample in our study is not a large sample that allows for multivariate modeling, it is large enough for exploratory analyses and meaningful description of changes in impairments that matter to patients before and after diagnosis of multiple myeloma. We obtained this sample using significantly less resources and time than would be required to recruit the same number of multiple myeloma patients. For example, a primary data collection study that lasted from 2012 to 2015 recruited only 40 older patients [5], while another study recruited 41 adult patients of all ages in 3 months [4]. Furthermore, by leveraging the longitudinal nature of the HRS, our study was able to obtain data on patient-reported measures of interest prior to diagnosis, which would be impossible to obtain using primary data collection. Our study shows that we can use alternative data sources, such as claims data or larger observational studies, to design and perform some basic, but important, health research studies on rare diseases.
Our study indicates significant decline in several patient-reported measures in multiple myeloma patients after diagnosis. First, our study shows that surviving patients experience significant functional decline after the diagnosis, as measured by difficulty with activities of daily living and mobility. Prior studies in older patients with other types of cancer have also shown the functional status declines after cancer diagnoses [24, 25]. While decline in those domains is common in ageing persons, we have also shown that the functional decline in multiple myeloma patients is significantly higher than decline in similar HRS participants without multiple myeloma diagnosis. Our study also shows that there is significant decline in patient well-being, as assessed by pain, self-reported health, and depression. Similar to functional decline, reports of pain, poor or fair self-rated health, and depressive symptoms become more common in surviving multiple myeloma patients after diagnosis than participants without multiple myeloma. These results are analogous to results of another study based on secondary longitudinal data source (English Longitudinal Study of Ageing), which showed that health and well-being of patient with cancer of any type deteriorated more rapidly than health and well-being of similar patient without cancer diagnosis [26]. Our findings are significant, since impairments in well-being can themselves lead to further bad outcomes in older patients [4, 27, 28].
The measures of function and well-being that we considered in this study are rarely targets of therapy in older persons with cancer and seldom used as outcome measures in clinical trials of treatments for myeloma and other cancers. Yet these measures are very important to cancer patients, some of whom view quality of life as a more important outcome than survival. The use of population-based data sources such as the HRS to determine which measures of function and well-being are mostly likely to deteriorate after a cancer diagnosis may be useful in helping designers of cancer treatment trials consider the types of outcome measures that might be included in their trials. Furthermore, both the high mortality rate of multiple myeloma patients (20% 1-year mortality) and the high likelihood of worsening quality of life in patients with multiple myeloma highlights the importance of holistic assessment and appropriate geriatric and palliative care. Palliative care has been recognized as an important part of care for cancer patients [29, 30], where patients’ physical, mental, and psychosocial needs need to be addressed in addition to treating the underlying cancer. Unfortunately, a prior study has shown that patients with hematological cancers are less likely to receive palliative care than patients with other cancers [31].
It is important to discuss several issues in this study that can limit the clinical conclusions of this and other studies using this type of secondary data. First, the time between multiple myeloma diagnosis and the interview before the diagnosis varies from 8 days to 34 months. Since patient-reported measures are not always measured immediately before the diagnosis, patient-reported measures might have changed for some patients between the last measurement and multiple myeloma diagnosis. Similarly, the time between the diagnosis and the interview after the diagnosis also varies. The patient-reported measures are likely to change often as the treatments progress, including repeated periods of improvement and decline [6]. Second, we do not have information on the treatments that each patient is receiving, which prevents us from understanding how different treatments affect the outcomes, and when in the treatment cycle the changes occur. Thus, our results further highlight the importance of including this important information about patient oriented outcomes in observational and clinical studies of cancer therapeutics.
Our study showed that secondary data can be used to identify patients with rare diseases, and perform exploratory and hypotheses generating studies. We showed that patient-reported measures in older adults worsen after multiple myeloma diagnosis, and the decline in surviving multiple myeloma patients is significantly worse than the decline is similar older adults without multiple myeloma. This indicates that older patients could benefit from supportive geriatric and palliative care to help manage the symptoms that worsen with multiple myeloma diagnosis and treatment.
References
Howlader N, Noone AM, Krapcho M et al (2017) SEER cancer statistics review, 1975-2014. National Cancer Institute, Bethesda, MD
Kumar SK, Dispenzieri A, Lacy MQ et al (2014) Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. https://doi.org/10.1038/leu.2013.313
Wildes TM, Rosko A, Tuchman SA (2014) Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. https://doi.org/10.1200/JCO.2014.55.1028
Kiely F, Cran A, Finnerty D et al (2017) Self-reported quality of life and symptom burden in ambulatory patients with multiple myeloma on disease-modifying treatment. Am J Hosp Palliat Med. https://doi.org/10.1177/1049909116646337
Wildes TM, Tuchman SA, Klepin HD et al (2018) Geriatric assessment in older adults with multiple myeloma. J Am Geriatr Soc 67:987–991. https://doi.org/10.1111/jgs.15715
King TA, King MT, White KJ (2017) Patient reported outcomes in optimizing myeloma patients’ health-related quality of life. Semin Oncol Nurs. https://doi.org/10.1016/j.soncn.2017.05.006
Lee SJ (2004) Patient-reported outcomes in multiple myeloma. JNCCN J Natl Compr Cancer Netw. https://doi.org/10.6004/jnccn.2004.0031
Health and Retirement Study. http://hrsonline.isr.umich.edu/. Accessed 15 Mar 2019
Elsawy B, Higgins KE (2011) The geriatric assessment. Am Fam Physician 83:48–56
Mariano J, Min LC (2012) Assessment. In: Naeim A, Reuben DB, Ganz PA (eds) Management of cancer in the older patient. Elsevier, Philadelphia, pp 39–50
Katz S, Ford AB, Moskowitz RW et al (1963) Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA, J Am Med Assoc. https://doi.org/10.1001/jama.1963.03060120024016
Hardy SE (2014) Consideration of function and functional decline. In: Williams BA, Chang A, Ahalt C et al (eds) Current diagnosis and treatment: geriatrics, 2nd edn. New York, McGraw-Hill Education
Smith AK, Cenzer IS, Knight SJ et al (2010) The epidemiology of pain during the last 2 years of life. Ann Intern Med. https://doi.org/10.7326/0003-4819-153-9-201011020-00005
Andrews JS, Cenzer IS, Yelin E et al (2013) Pain as a risk factor for disability or death. J Am Geriatr Soc. https://doi.org/10.1111/jgs.12172
Persons AGSP on the PM of PP in O (2009) Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 57:1331–1346. https://doi.org/10.1111/j.1532-5415.2009.02376.x
Swarm R, Abernethy AP, Anghelescu DL et al (2010) Adult cancer pain. J Natl Compr Canc Netw 8:1046–1086. https://doi.org/10.6004/jnccn.2010.0076
Lynn J, Teno JM, Phillips RS et al (1997) Perceptions by family members of the dying experience of older and seriously ill patients. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. Ann Intern Med 126:97–106. https://doi.org/10.7326/0003-4819-126-2-199701150-00001
Turvey CL, Wallace RB, Herzog R (1999) A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. https://doi.org/10.1017/S1041610299005694
Steffick DE (2000) Documentation of affective functioning measures in the health and retirement study. Institute for Social Research, University of Michigan, Ann Arbor
SAS Institute (2016) The SAS system for Windows. Release 9.4. SAS Inst., Cary, NC
Ho DE, Imai K, King G et al (2015) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. https://doi.org/10.18637/jss.v042.i08
R Core Team (2017) R: A language and environment for statistical computing. https://www.r-project.org/
Costa LJ, Gonsalves WI, Kumar SK (2015) Early mortality in multiple myeloma. Leukemia. https://doi.org/10.1038/leu.2015.33
Kenis C, Decoster L, Bastin J et al (2017) Functional decline in older patients with cancer receiving chemotherapy: a multicenter prospective study. J Geriatr Oncol. https://doi.org/10.1016/j.jgo.2017.02.010
Decoster L, Kenis C, Schallier D et al (2017) Geriatric assessment and functional decline in older patients with lung cancer. Lung. https://doi.org/10.1007/s00408-017-0025-2
Williams K, Jackson SE, Beeken RJ et al (2016) The impact of a cancer diagnosis on health and well-being: a prospective, population-based study. Psychooncology. https://doi.org/10.1002/pon.3998
Bruera E, Schoeller T, Wenk R et al (1995) A prospective multicenter assessment of the Edmonton staging system for cancer pain. J Pain Symptom Manag. https://doi.org/10.1016/0885-3924(95)00052-Z
Bamia C, Orfanos P, Juerges H et al (2017) Self-rated health and all-cause and cause-specific mortality of older adults: individual data meta-analysis of prospective cohort studies in the CHANCES Consortium. Maturitas. https://doi.org/10.1016/j.maturitas.2017.06.023
Zanwar S, Abeykoon JP, Kapoor P (2019) Challenges and strategies in the management of multiple myeloma in the elderly population. Curr Hematol Malig Rep. https://doi.org/10.1007/s11899-019-00500-4
Ferrell BR, Temel JS, Temin S et al (2017) Integration of palliative care into standard oncology care: American society of clinical oncology clinical practice guideline update. J Clin Oncol. https://doi.org/10.1200/JCO.2016.70.1474
Howell DA, Shellens R, Roman E et al (2011) Haematological malignancy: are patients appropriately referred for specialist palliative and hospice care? A systematic review and meta-analysis of published data. Palliat Med. https://doi.org/10.1177/0269216310391692
Acknowledgments
Open Access funding provided by Projekt DEAL.
Funding
Dr. Kenneth Covinsky is supported by the UCSF Older Americans Independence Center (P30 AG044281).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Irena Cenzer, Annette Rodriguez, and Kenneth Covinsky. The first draft of the manuscript was written by Irena Cenzer and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UCSF Institutional Review Board (IRB), IRB number 10-00883) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Written informed consent was provided by participants before entering the HRS.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cenzer, I., Berger, K., Rodriguez, A.M. et al. Patient-reported measures of well-being in older multiple myeloma patients: use of secondary data source. Aging Clin Exp Res 32, 1153–1160 (2020). https://doi.org/10.1007/s40520-019-01465-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-019-01465-3