Abstract
Background and aims
Dual-task walking (DTW) is thought to involve activation of the prefrontal cortex in healthy adults and to be affected by cognitive impairment. However, it is unclear whether prefrontal cortex activation is involved in DTW in older adults with mild cognitive impairment. This study examined brain activation during DTW among older adults with mild cognitive impairment using functional near-infrared spectroscopy.
Methods
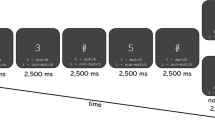
Sixteen older adults (aged 75.4 ± 7.2 years, women n = 6) performed gait experiments under normal walking and DTW conditions. We used a design with 60-s blocks consisting of a 10-s rest standing as pre-resting period, a 20-s walking task period, and a 30-s rest standing as post-resting period. Walking speed was measured during a 20-s walking task. Changes in oxy-hemoglobin were measured in the prefrontal area during gait experiments.
Results
Walking speed was slower during DTW compared with normal walking (p < 0.001). The oxy-hemoglobin level during DTW was higher than during normal walking (p < 0.001) and was correlated with executive function, as measured by Stroop interference (p < 0.05).
Conclusion
Our findings indicate that DTW is associated with prefrontal activation among older adults with mild cognitive impairment. The brain activation during DTW was correlated with executive function. Additional studies are necessary to elucidate the effects of cognitive impairment on the association between prefrontal activity and walking under various conditions.


Similar content being viewed by others
References
Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J (2011) Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev 35:715–728
Woollacott M, Shumway-Cook A (2002) Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16:1–14
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23: 329–342 (quiz 472)
Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N (2008) Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci 63:1335–1343
Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM (2010) Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci 65:1086–1092
Sheridan PL, Solomont J, Kowall N, Hausdorff JM (2003) Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc 51:1633–1637
Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M (2012) Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture 35:96–100
Pettersson AF, Olsson E, Wahlund LO (2005) Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord 19:299–304
Gillain S, Warzee E, Lekeu F et al (2009) The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Ann Phys Rehabil Med 52:453–474
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194
Verghese J, Wang C, Lipton RB, Holtzer R, Xue X (2007) Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 78:929–935
Yamada M, Tanaka B, Nagai K, Aoyama T, Ichihashi N (2010) Trail-walking exercise and fall risk factors in community-dwelling older adults: preliminary results of a randomized controlled trial. J Am Geriatr Soc 58:1946–1951
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) Fnirs study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879–887
la Fougere C, Zwergal A, Rominger A et al (2010) Real versus imagined locomotion: a f-18 -fdg pet-fmri comparison. Neuroimage 50:1589–1598
Hanakawa T, Katsumi Y, Fukuyama H et al (1999) Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain 122(Pt 7):1271–1282
Miyai I, Tanabe HC, Sase I et al (2001) Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14:1186–1192
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Makizako H, Shimada H, Doi T et al (2011) The association between decline in physical functioning and atrophy of medial temporal areas in community-dwelling older adults with amnestic and nonamnestic mild cognitive impairment. Arch Phys Med Rehabil 92: 1992–1999
Alvarez JA, Emory E (2006) Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev 16:17–42
Kato M (2001) Prefrontal lobes and the attentional control: a neuropsychological study using modified stroop test. Clin Neurol (Rinsho shinkeigaku) 41:1134–1136
Takahashi T, Takikawa Y, Kawagoe R, Shibuya S, Iwano T, Kitazawa S (2011) Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage 57:991–1002
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol Ser A-Biol Sci Med Sci 66:879–887
Wobst P, Wenzel R, Kohl M, Obrig H, Villringer A (2001) Linear aspects of changes in deoxygenated hemoglobin concentration and cytochrome oxidase oxidation during brain activation. Neuroimage 13:520–530
Kita Y, Gunji A, Inoue Y et al (2011) Self-face recognition in children with autism spectrum disorders: a near-infrared spectroscopy study. Brain Dev 33:494–503
Yamamoto T, Kato T (2002) Paradoxical correlation between signal in functional magnetic resonance imaging and deoxygenated haemoglobin content in capillaries: a new theoretical explanation. Phys Med Biol 47:1121–1141
Strangman G, Franceschini MA, Boas DA, Yamamoto T, Kato T (2003) Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters paradoxical correlation between signal in functional magnetic resonance imaging and deoxygenated haemoglobin content in capillaries: a new theoretical explanation. Neuroimage 18:865–879
Verghese J, Robbins M, Holtzer R et al (2008) Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 56:1244–1251
Leon-Carrion J, Damas-Lopez J, Martin-Rodriguez JF et al (2008) The hemodynamics of cognitive control: the level of concentration of oxygenated hemoglobin in the superior prefrontal cortex varies as a function of performance in a modified stroop task. Behav Brain Res 193:248–256
Schroeter ML, Zysset S, Wahl M, von Cramon DY (2004) Prefrontal activation due to stroop interference increases during development–an event-related fNIRS study. Neuroimage 23:1317–1325
Dickerson BC, Sperling RA (2008) Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46:1624–1635
Acknowledgments
We would like to thank the Obu city office for help with participant recruitment. This work was supported by a grant from the Japanese Ministry of Health, Labour and Welfare and Grant-in-Aid for Research Activity Start-up (22800093) to T.D. in Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doi, T., Makizako, H., Shimada, H. et al. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res 25, 539–544 (2013). https://doi.org/10.1007/s40520-013-0119-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-013-0119-5