Abstract
Purpose of Review
Neuropathic pain (NP) has been ubiquitously characterized by lesion and its linked somatosensory system either the central nervous system (CNS) or peripheral nervous system (PNS) This PNS episode is the most prevalent site of NP origin and is found to be associated with afferent nerve fibers carrying pain signals from injured/trauma site to the CNS including the brain. Several kinds of pharmacotherapeutic drugs shuch as analgesics, anti-convulsants, and anti-depressants are being employed for the its possible interventions. The NP has been a great interest to follow different pathophysiological mechanisms which are often considered to correlate with the metabolic pathways and its mediated disease. There is paucity of knowledge to make such mechanism via NP.
Recent Finding
Most notably, recent pandemic outbreak of COVID-19 has also been reported in chronic pain mediated diabetes, inflammatory disorders, and cancers. There is an increasing incidence of NP and its complex mechanism has now led to identify the possible investigations of responsible genes and proteins via bioinformatics tools. The analysis might be more instrumental as collecting the genes from pain genetic database, analyzing the variants through differential gene expression (DEG) and constructing the protein–protein interaction (PPI) networks and thereby determining their upregulating and downregulating pathways.
Summary
This review sheds a bright light towards several mechanisms at both cellular and molecular level, correlation of NP-mediated disease mechanism and possible cell surface biomarkers (receptors), and identified genes could be more promising for their pharmacological targets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
NP has been known as a pain caused by lesion or diseases affecting the somatosensory system, which then interferes with the perception of touch, pressure, and pain and arises from different parts as joints, muscles, and fascia, including various sensory receptors [1]. At a global level, it has been highlighted as approximately 7–10% and varies at various mechanical and hyperalgesia degrees [2]. The onset of NP is mostly related with metabolic disorders such as diabetes, viral infection-linked neuropathies, and autoimmune and inflammatory disorders including rheumatoid arthritis [3.••]. It has been investigated that any 7damage to the peripheral nerves results in peripheral neuropathic pain and has been associated with AIDS, multiple sclerosis, stroke, and other chronic disorders, whereas central neuropathic pain arise from CNS injury [4]. The prognosis of NP could be performed by screening for reflexes, sensory, and motor function via imaging techniques as MRI, CT, and X-rays [5, 6].
In recent years, available pharmacotherapy against neuropathic pain has been categorized into four major lines as first-line interventions used as serotonin-noradrenaline inhibitor (e.g., pregabalin drug), second-line interventions that allows a regulated opioid release, third-line treatment as cannabinoids, and fourth-line treatment as botulinum toxin, methadone [7.•]. Such medications and/or therapy are more capable to target receptors including neuroimmune cells triggers the pain as a result of immune response. In 2019, Oliveira-Fusaro reported that NP initiation and maintenance require crucial signaling from microglia to neurons that is critically linked with the upregulation of P2X4 receptor [8.••].
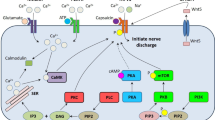
In addition, RNA research has been focused on differentially expressed mRNA and miRNA and has reported the genes CXCR2, GRK1, IL12B, and TNFSF8 are involved in the pathogenesis of NP [9]. Owing to genetic abnormalities or mutations, very limited cases of NP are reported at clinical level. For example, Fabry disease has been known for the mutation in the X- chromosome and imparts its more effects on males at higher rate than the females [10]. Unlike targets identification, release of inflammatory and/or cytokines have also contributed to peripheral nerve damage. These NP mediated cytokines could also considered as both pro-and anti-inflammatory molecules. Promisingly, displayed to be the crucial players for triggering pain and its mediated activated immune machineries (Fig. 1) [11].
Several animal and/or rodent models have shown a great interest for pain researches and utilized for the possible drug-targeted therapies in NP mechanism at both PNP and CNP systems. These experimental research models have also been implied to enquire the efficacy of the developed pharmacological drugs/molecules for therapeutic interventions as either non-invasive or non-pharmacological stage [12.•]. This review will provide an insight and shed a bright light on the available treatment options for neuropathic pain in mediated COVID-19 infections and other complications at both pre-clinical and clinical platform.
Characterization and Mechanistic Episodes of NP
Due to lesions in the spinal cord and/or brain, central neuropathic pain could be an integral action linked with syringomyelia and demyelinating diseases. In contrast, the unmyelinated C fibers have been considered as small in size and myelinated A fibers including Aβ and Aδ fibers have also been known to play a key role in the pathology of peripheral neuropathic pain. Such fibers have been recognized as sensory fibers [1]. Both central and peripheral neuropathic pain arise at the site of skin with abnormal sensation. Sensory fibers have been considered for diagnostic approach and critically linked with both positive and negative sensory symptoms in patients. In addition, pain has been detected maximally at particular site via increased mechanical hypersensitivity and reduced hypersensitivity to thermal sensation as heat and cold. Altered and disoriented sensory signal transmissions are the resultants of affected nerves with alterations in their ion channels which includes, increased sodium ion channel activity enhancing the excitability and poor fluxes of potassium ions [13]. The nociceptive signaling has also been influenced by the tunneling release of neurotransmitters that are mediated by voltage-gated calcium channel (VGCC) and any dysregulation and/or dysfunction to the voltage-gated sodium channels (VGSC) also raised the sodium ion channel activity and thereby resulting hyperalgesia with non-painful stimuli [14]. This electrifying potential is needed to activate VGSC that is mediated by the interaction of pain stimuli molecules to the transient receptor potential (TRP). It has also a major concern for mechanical stimuli. Under pathological/physiological conditions, such kind of stimuli could show an activation of TRPA1 and TRPV4. It has also observed that both hot and cold temperature triggered thermal stimului are more capabale to activate TRPA1, TRPM8, and TRPV1 respectively (Fig. 2) [15].
It has been implicated that any painful sensation or pain induced by any pathological condition alters the microglia and astrocyte function which then further modify the spinal microenvironment and therefore causing the release of soluble neurotransmitter molecules. Such molecules or neurotransmitters are able to show nociceptive neuronal excitation and helps for the excitation of Na+ channel excitation [16]. Additionally, an injury to the peripheral afferent nerves or peripheral axotomy causes invasion of immune cells at the juncture of dorsal root ganglions (DRGs). These actions have shown an extended release of cytokines and multiple alterations in gene expression, thus considering as the primary site of neuropathic pain at the injury site [17]. The chemokines which set a positive feedback loop in neuroinflammatory signal cascade are CCL2, CX3CL1, and CXCL1 [18.••]. The persistent changes in the gene expression have also found with an involvement of neuroimmune interactions. Some of the recognized genes involved in neuropathic pain are Il1 receptor antagonist (Il1rn and other immune genes [19]. Once the P2X (P2X1, P2X2, P2X3, P2X4, P2X5 P2X6, and P2X7) receptor binds with ATP, opening of pore cascade initiated to produce purinergic signaling at patho/physiological cconsequences). Therefore, the upregulation of P2X4 triggers the release brain derived neurotrophic factors (BDNF) causing variation in membrane anion gradient in NP [20].
NP-Mediated Inflammatory Diseases and Its Pharmacological Interventions
Diabetic Neuropathy
Diabetic peripheral neuropathy (DPN) is commonly represented as distal symmetric polyneuropathy (DSPN) and the foremost neuropathy distributed at global level [21]. Sensory, autonomic, and motor axons are the preferred targets in the neurodegenerative diseases of the PNS. Axon-Schwann cell interaction is essential for maintenance of axon-cytoskeletal structure. It has been depicted that any damage to the Schwann cell results in axonal degeneration. Importantly, mononucleotide adenylyltransferase has been regarded as crucial player for diabetic neuropathy mechanisms [22]. Blood-nerve barrier (BNB) has been known for maintaining the intact parts of nervous system. Any damage due to injury or pathological condition may alter the delicate BNB drastically and caused high permeability in BNB and considered as the first reported step for development of diabetic neuropathy [23.•]. Reports have also published on other regulators (glucose and insulin) for controlling the multiple cellular processes in brain. Both the hypoglycemic and hyperglycemic conditions could also show diabetic neuropathic pain (DNP) modulation in the central nervous system [24].
Cancer
Neuropathic cancer pain has also shown an increasing recognition and displayed either primary tumor/metastases or due chemotherapeutic interventions that affects the nervous system. The primary state of such conditions occurs due to the infiltration of tumor to the different parts of the PNS or CNS [25]. At the initial stage, the tumors would infiltrate the nerves thereby disrupting the neuronal transmission. This is accompanied by the inflammation and further infiltration mediated differentiation in nerve cells [26.•]. Whereas in final stage, chemotherapy-induced neuropathic pain (CINP) has been shown a negative impact on PNS via chemotherapeutic drugs as paclitaxel, and oxaliplatin has been reported with major incidences [27]. Initial chemotherapeutic treatment produces acute pain followed by repetitive treatment cycles to cause chronic neuropathic pain [28].
Chronic Inflammatory Rheumatoid Diseases (CIRD)
Rheumatic disease has been revolutionized and/or characterized by fibromyalgia and considerably involves inflammatory pain which leads to the impaired function of cellular systems. It has been evidenced that rheumatic diseases chronic episodes as rheumatoid (RA), psoriatic (PsA), and spondyloarthritis (SpA), where SpA is reported to have high prevalence of neuropathic pain [29]. The detection of such CIRD has put to enquire Pain DETECT questionnaire [30] and DN4 questionnaire [31]. On the contrary, fibromyalgia was shown with a lack of lesions in the somatosensory system while similar to central neuropathic pain (CNP) and has been reported with the modulation in the levels of neurotransmitter signaling and thereby causing small fiber neuropathies [32.•].
Central Post-Stroke Pain
Central post-stroke pain (CPSP) is a chronic neuropathic pain due to CNS damage characterized by dysfunction of spinothalamic tract (ascending pathway that transmits pain, temperature, and senses from spinal cord), central sensitization, and pain network disinhibition [33]. The pathophysiology of thalamic pain syndrome with lesion in the CNS is complicated with contributions from cerebral cortex, opioid receptor, and long-chain fatty acid receptor GPR40 [34]. When it comes to the therapeutic intervention for CPSP drugs like pregabalin, gabapentin and amitriptyline and mirror therapy (MT) were preferred over physiotherapy which is less responsive [35.•].
Alzheimer’s Disease and Neuropathic Pain
Alzheimer’s disease (AD) is a cerebral cortex disease which is characterized by cognitive abnormalities and emotional dysfunction [36]. Patients with such cognitive abnormality may not be able to communicate their pain and seek proper treatment. The correlation study between chronic pain and AD has suggested pathologies like microglial activation, noradrenergic system abnormality, and increased central neuroinflammation [37].
Central Neuropathic Pain in Parkinson’s Disease
The most common neurodegenerative disorder among the geriatric population is Parkinson’s disease (PD); associated with motor dysfunction, it often remains unnoticed. James Parkinson describes the varying pain from early stage to advanced stage as Parkinson agitans which includes both neuropathic (radicular or articular) and nociceptive pain [38]. Chronic pain in PD is assessed using different questionnaires like Leeds Assessment of Neuropathic Symptoms and Signs, Brief pain inventory, and King’s PD pain scale, which classifies the various pain and broadly categorize them as nociceptive, neuropathic, and miscellaneous pain [39].
Traumatic Brain Injury and Painful Episodes
External force or exposure to the head either due to direct blow or shock wave is known as traumatic brain injury (TBI) with an annual incidence of 50 million cases and the most prevalent being the mild TBH which affects 75–80% of the population which often resulted in chronic posttraumatic headache (PTH) [40, 41]. The pathophysiologic investigations on the brains of patients who sustained TBI have revealed symptoms related to Alzheimer’s, which is given as chronic traumatic encephalopathy. TBI occurs when the brain hits the surface of the cranium as a result of force applied, and this causes tearing of axons in the white matter, known as “diffuse axonal injury” [42].
Brain Tumors and Neuropathic Pain
The nerve which is infiltrated or compressed by tumor affects the nervous system directly and often leads to neuropathic cancer pain (NCP) [43].
COVID-19 Infection and Neuropathic Pain
In recent years, COVID-19 or human corona virus was found to be more neuroinvasive which then possibly could show axonal propagation. Such events have been symptomatically reported with muscular and joint pain followed by headache [44.••]. The invasion of this magic virus was enquired in biological fluid as CSF in COVID-19-infected patients and the confirmation of its presence could be detected by using RT-PCR. This manifestation has been progressive which could be due to the direct attack of virus on nervous system, due to parainfection (infectious agents affect the nervous system causing direct illness, meningitis) or post- infection at the time of recovery, where immunity has been compromised [45]. In the absence of an impaired electrophysiological state, small fiber-mediated neuropathies were observed in COVID-19-infected patients involving both the central and peripheral nervous system and have led to the suspect of neurotropism [46].
Biomarkers and Pharmacological Targets on Neuropathic Pain
Neuropathic pain has been investigated with its specific biomarkers and /or receptors pharmacological drugs. In 2018, Davis has reported the relationship between NP and epigenetics and conveyed message as neuropathic injury causes the abnormalities in miRNA expression and is capable to show downregulation of the potassium ion channels and glutamate transporters [47]. This has led the elevated expression of trophic factors including brain-derived neurotrophic factor. Clusters of miRNAs have also been established and considered via reduced expressions as miR-182, -183, and -96 in the dorsal root ganglions (DRG) of spinal nerve ligation (SNL) [48]. Epigenetic modifications have also been studied and made possible contributions with pain transition from acute to chronic state. One such modification is being conferred as DNA methylation in DRG due to peripheral nerve injury or SNL and has shown alterations in the expression of DRG gene [49]. Another epigenetic modification as histone methylation was also deciphered wherein the expression and/or levels of chemokines and mRNA modifications were raised in NP due to peripheral nerve injury.
On contrary, histone deacetylases (HDAC) were also reported to alleviate the neuropathic pain [50]. Neuronal injury or damage could also reported with the activation of peptidogenic or non-peptidogenic fibers or the peripheral afferent fibers including Aδ and C. This could be induced with the release of glutamate followed by the activation of G- protein coupled receptors (GPCR) that help to trigger the surrounding immune cells and could be considered as potential target [51]. In addition, synaptic transmissions are being mediated by the glutamate amino acid. This transmission episode could either be rapid or slow via its specific receptors as AMPARs or NMDARs, respectively. This scenario was reported and shown critical link between central sensitization and pain hypersensitivity [51]. It has been known that the most abundant and excitatory neurotransmitter was considered as glutamate and it has recognized on pain signaling cascades either via metabotropic or ionotropic factors. Unlike neurons, metabotropic glutamate receptors (mGluR) could be observed in glial cells prominently in astrocytes, microglia, and oligodendrocyte [52].
The glial cells have been implicated as immune cells in the central nervous system. In male population, the neuropathic pain was predominantly caused by microglia and on the contrary, female population, pain was observed due to the infiltration of T-lymphocytes [53].
In neuropathic pain animal model, several genetic markers have also identified and critically linked via genome-wide association investigations. It has also been reported that the genes could involve in neuropathic pain and thereby interrelated with metabolic process and ion channels. Several kinds of genes including MPZ, PRKCA, GCH1, COMT, and SCN11A have been identified in neuropathic studies in in vivo research models [54]. Another investigation has contributed to human pain genetics database and evidenced the variants single gene associated with monogenic disorders. These disorders have been recognized with pain machinery components as erythromelalgia wherein a variant gene SCN9A was reported to modulate the DRG electrophysiological properties [55]. In 2017, Chen and his co-workers have conducted one study and suggested that the non-injured or normal dorsal root ganglions adjacent to the injured and/or impaired DRGs could be contributed to the neuropathic pain. On this platform, the genes and their specific signaling cascade could be involved wherein FoxO played a key role for cell maintenance, Ctnnb1 for Wnt signaling, and Wt1 DEG and PPI networks.
In Vitro Research Models for Neuropathic Pain
Peripheral sensory neurons (PSN) are responsible to discriminate the different senses as they are heterogeneous in population. Direct collection of human PSN is not possible, so they are derived from human embryonic stem cells using the small molecule inhibition method. As they are believed to mimic the in vivo pathophysiology, they are used in in vitro peripheral nerve injury study [56]. Human fibroblasts were reprogrammed to nociceptor neuron (first order pain sensory detection pathway) using different transcription factors like Ascl1 and Brn2 which generate neuron of generic type. Nociceptor neurons further develop into neuropathic pain and become sensitive to chemotherapeutic drugs or inflammatory mediators like cytokin [57]. The therapeutic drugs developed to treat peripheral nerve injuries (PNI) are subjected to in vitro model screening which uses excised spinal column from Sprague Dawley rats for dorsal root ganglion (DRG) neurons [58]. Engineering an in vitro model for CNS is difficult because of the lack of pathophysiological information like twist, compression, and physiological resemblance, which is overcome by 3D neuronal-glial model that mimics human head injuries mainly TBI and other CNS diseases [59].
In Vivo Research Models for Neuropathic Pain
Seltzer Model
In 1990, Seltzer and his co-workers have pointed out and revealed an excellent behavior model for evaluating neuropathic pain deficit in rats and considered for partial schiatic nerve injury [60]. This modality is based on a ligation at the juncture of the ipsilateral sciatic nerve. This nerve could be detected at hind paw of the experimental rats. Within few hours of partial schiatic nerve injury, the experimental rats could show allodynia, whiping, instinctive pain, and hyperalgesia. These kinds of behavior were observed at both (thermal and mechanical) the ligation process.
Spinal Nerve Ligation at L5/L6 Position
This model has not been deciphered yet at proper stage. It has been considered as an easy technique to perform L5/L6 ligation. The experimental rats could behave and depict a prolonged signals of both mechanical allodynia and hyperalgesia. Some reports have suggested and speculated for its consideration of modality in mice.
Axotomy model
This technique is well recognized and more considerable model for provoking neuropathic pain. It has also been used to poke a transection at the entire sciatic nerve present at the mid region of hind paw. In this techniques, the targeted sciatic nerve is dislocated from the connective tissue. Furthermore, a tight ligation is done by a nylon made suture that should be in a proximity at a juncture of both tibial and peroneal ways. This can be located with a gap of 1 cm. The nerve is therefore perfectly transected at the surrounded environment of suture invoked ligatures.
Spared Nerve Injury
This is also a more advanced neuropathic pain animal model established by Decosterd and Woolf. In this particular model, the experimental animals and/or rats were anesthetized followed by the dissection via biceps femoris muscles. In context of the spared nerve injury, sciatic nerve and its considered terminal branches as sural, common peroneal, and tibial were targeted. Thereafter, the tibial along with common peroneal nerves was tightly ligated and thereby ensured with an axotomical episodes at a distance of 2 mm on distal nerve juncture.
Pharmacological Guidelines and Treatment for NP
For the past decade, the European Federation of Neurological Societies (EFNS) has announced the primary guidelines on pharmacological intervention against neuropathic pain. The management of NP highlights on targeting the symptoms and limited only in some of the pathological condition. There is one recent report based on drug effectiveness wherein a particular intention on NP was proposed gabapentinoids and tricyclic antidepressants (TCAs), and serotonin–norepinephrine reuptake inhibitors were considered as first-line drugs against NP. In addition, lidocaine, capsaicin, and tramadol have been suggested as the second-line treatment, while morphine and oxycodone and botulinum toxin-A were recommended as third-line treatments for neuropathic pain in peripheral. Likewise, gabapentin and pregabalin were also approved by FDA for the therapeutic intervention of NP.
Spinal Cord and Traumatic Brain Injury-Mediated Pain Mechanism
TBI has been linked with both acute and chronic pain in animal models and humans. At initial stage, trauma evokes a somatic pain and considered as secondary injury machinary. This has made a possible contribution for both visceral and neuropathic pain. Pharmacological pain management immediately following experimental TBI in animal models can influence post-injury outcomes (Table 1). In addition, several animal models mimic SCI mediated pain. The spinal cord may be damaged directly via contusion, surgical lesions of the cord, and neurotransmitter-provoked excitotoxicity. Such kind of spinal cord models could be resulted as in mechanical and thermal (heat and cold) hyperalgesia.
Therapeutic Interventions Against Neuropathic Pain and Its Management
Several therapeutic strategies are developed for the treatment and management of neuropathic pain. The pharmacological interventions are classified into three different categories based on their activity like serotonin uptake inhibitors, TCAs, opioids, and antiepileptic drugs. The identification of several biomarkers is found to be promising for future biomarker-based pain therapy which requires correlation-based information on biomarker and pharmacological effect, yet it is still time consuming and expensive. Non-pharmacological interventions are often non-invasive and the different therapies include mirror, cognitive behavior, adjunctive, and animal-assisted [61]. Non-destructive method followed after the failure of traditional treatments includes radio frequency (RF) approach which is categorized into pulsed and continuous RF of which pulsed RF is the most preferred as they modulate the pain signals instead of blocking them [62].
Conclusion
Neuropathic pain is chronic and has an increasing importance to enquire the negative impacts in population at global level. A diverse range of pathophysiological mechanism is being interconnected with other metabolic pathways in disease state. Considering the recognition of neurological disturbances in COVID-19 infections, we also convey a message that COVID-19-infected patients always have complaints for neuropathic pain. The exact mechanism of neuropathic pain is yet to be discovered, many pharmacogenomics studies and bioinformatics analyses are being deciphered, and several genes and proteins are also in debate for the identification and targets the patients with personalized medicines. Animal model development has also raised an understanding for pain and its linked mechanisms. In addition, pain models owing to pharmacological drugs and other diseases medications have also been recognized with better consideration of their pathophysiology and management.
Data availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN. Neuropathic pain Nat Rev Dis Primers. 2017;3:17002.
Liu H, Xia T, Xu F, Ma Z, Gu X. Identification of the key genes associated with neuropathic pain. Mol Med Rep. 2018;17(5):6371–8.
Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:2058738419838383. It covers the pharmacological therapeutic episodes against neuropathic pain.
Khangura RK, Sharma J, Bali A, Singh N, Jaggi AS. An integrated review on new targets in the treatment of neuropathic pain. Korean J Physiol Pharmacol. 2019;23(1):1–20.
Masuda R, Ajimi J, Murata T. Pharmacotherapy for neuropathic pain in Japan. J Nippon Med Sch. 2017;84(6):258–67.
Nascimento OJ, Pessoa BL, Orsini M, Ribeiro P, Davidovich E, Pupe C, Filho PM, Dornas RM, Masiero L, Bittencourt J, Bastos VH. Neuropathic pain treatment: still a challenge. Neurol Int. 2016;8(2):6322.
Alles SRA, Smith PA. Etiology and Pharmacology of neuropathic pain. Pharmacol Rev. 2018;70(2):315–47. This article consists the probable action of drug and/or molecules for targeting neuropathic pain.
Oliveira-Fusaro MCG. New promising targets to control neuropathic pain. Pain. 2019;160(9):1907–8. It covers the considerable cellular targets for the regulation of pain.
Li H, Wan HQ, Zhao HJ, Luan SX, Zhang CG. Identification of candidate genes and miRNAs associated with neuropathic pain induced by spared nerve injury. Int J Mol Med. 2019;44(4):1205–18.
Klein MC, Oaklander AL. Ion channels and neuropathic pain. Elife. 2018;7:e42849.
Hung AL, Lim M, Doshi TL. Targeting cytokines for treatment of neuropathic pain. Scand J Pain. 2017;17:287–93.
Rice ASC, Finnerup NB, Kemp HI, Currie GL, Baron R. Sensory profiling in animal models of neuropathic pain: a call for back-translation. Pain. 2018;159(5):819–24. In this article, investigations of rodent model in cellular mediated neuropathic pain.
Bannister K, Sachau J, Baron R, Dickenson AH. Neuropathic pain: mechanism-based therapeutics. Annu Rev Pharmacol Toxicol. 2020;60:257–74.
Chew LA, Khanna R. CRMP2 and voltage-gated ion channels: potential roles in neuropathic pain. Neuronal Signal. 2018;2(1):NS20170220.
Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28.
Vicario N, Turnaturi R, Spitale FM, Torrisi F, Zappalà A, Gulino R, Pasquinucci L, Chiechio S, Parenti C, Parenti R. Intercellular communication and ion channels in neuropathic pain chronicization. Inflamm Res. 2020;69(9):841–50.
Esposito MF, Malayil R, Hanes M, Deer T. Unique characteristics of the dorsal root ganglion as a target for neuromodulation. Pain Med. 2019;20(Suppl 1):S23–30.
Fernandes V, Sharma D, Vaidya S, Guan Y, Kalia K, Tiwari V. Cellular and molecular mechanisms driving neuropathic pain: recent advancements and challenges. Expert Opin Ther Targets. 2018;22(2):131–42. It confers the considerable episodes of neuropathic pain and future paradigm of such particular interest at global level.
Zakin E, Abrams R, Simpson DM. Diabetic neuropathy. Semin Neurol. 2019;39(5):560–9.
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41.
Richner M, Ferreira N, Dudele A, Jensen TS, Vaegter CB, Gonçalves NP. Functional and structural changes of the blood-nerve-barrier in diabetic neuropathy. Front Neurosci. 2019;12:1038.
Malone JI. Diabetic central neuropathy: CNS damage related to hyperglycemia. Diabetes. 2016;65(2):355–7.
Edwards HL, Mulvey MR, Bennett MI. Cancer-related neuropathic pain. Cancers (Basel). 2019;11(3):373. In this article, cancer mechanism has been recognized as a culprit episode for provoking neuropathic pain..
Loomba V, Kaveeshvar H, Upadhyay A, Sibai N. Neuropathic pain in cancer patients: a brief review. Indian J Cancer. 2015;52(3):425–8.
Gilchrist LS, Tanner LR, Ness KK. Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer. 2017;64(1):180–7.
Colvin LA. Chemotherapy-induced peripheral neuropathy: where are we now? Pain. 2019;160(Suppl 1):S1–10. This article suggests the action of chemotherapeutic drugs on cancer patients and induction of peripheral neuropathy and/or neuropathic pain..
Mathieu S, Couderc M, Pereira B, Dubost JJ, Malochet-Guinamand S, Tournadre A, Soubrier M, Moisset X. Prevalence of migraine and neuropathic pain in rheumatic diseases. J Clin Med. 2020;9(6):1890.
Garip Y. Prevalence of neuropathic pain in rheumatic disorders: association with disease activity, functional status and quality of life. Arch Rheumatol. 2015;30(3):231–7.
Narayan RV, Thabah MM, Poduval M. Neuropathic pain among patients with primary knee osteoarthritis: results of a cross-sectional study from a tertiary care center in Southern India. Indian J Rheumatol. 2017;12:132–8.
Cheng CW, Wong CS, Hui GK, Chung EK, Wong SH. Fibromyalgia: is it a neuropathic pain? Pain Manag. 2018;8(5):377–88.
Tang SC, Lee LJ, Jeng JS, Hsieh ST, Chiang MC, Yeh SJ, Hsueh HW, Chao CC. Pathophysiology of central poststroke pain: motor cortex disinhibition and its clinical and sensory correlates. Stroke. 2019;50(10):2851–7.
Treister AK, Hatch MN, Cramer SC, Chang EY. Demystifying Poststroke Pain: From Etiology to Treatment. PM R. 2017;9(1):63–75. This article recommends the probable treatment or therapeutic interventions against later stages of stroke mediated pain..
Corbetta D, Sarasso E, Agosta F, Filippi M, Gatti R. Mirror therapy for an adult with central post-stroke pain: a case report. Arch Physiother. 2018;8:4.
Soria Lopez JA, González HM, Léger GC. Alzheimer’s disease. Handb Clin Neurol. 2019;167:231–55.
Cao S, Fisher DW, Yu T, Dong H. The link between chronic pain and Alzheimer’s disease. J Neuroinflammation. 2019;16(1):204. It envelops the cross talk and possible reason for induction of pain in inflammation mediates Alzheimer’s disease..
Moreno CB, Hernández-Beltrán N, Munévar D, Gutiérrez-Alvarez AM. Central neuropathic pain in Parkinson’s disease. Neurología (English Edition). 2012;27(8):500–3.
Blanchet PJ, Brefel-Courbon C. Chronic pain and pain processing in Parkinson’s disease. Prog Neuropsychopharmacol Biol Psych. 2018;87(Pt B):200–6.
Niu X, Bai L, Sun Y, Wang Y, Bai G, Yin B, Wang S, Gan S, Jia X, Liu H. Mild traumatic brain injury is associated with effect of inflammation on structural changes of default mode network in those developing chronic pain. J Headache Pain. 2020;21(1):135.
Irvine KA, Clark JD. Chronic pain after traumatic brain injury: pathophysiology and pain mechanisms. Pain Med. 2018;19(7):1315–33.
Dixon KJ. Pathophysiology of traumatic brain injury. Phys Med Rehabil Clin N Am. 2017;28(2):215–25.
Yoon SY, Oh J. Neuropathic cancer pain: prevalence, pathophysiology, and management. Korean J Intern Med. 2018;33(6):1058–69.
Aksan F, Nelson EA, Swedish KA. A COVID-19 patient with intense burning pain. J Neurovirol. 2020;26:800–1.
Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–83.
Kemp HI, Corner E, Colvin LA. Chronic pain after COVID-19: implications for rehabilitation. Br J Anaesth. 2020;125(4):436–40. This article shows the recent probe of pain in post COVD-19 infections..
Davis MP. Cancer-related neuropathic pain: review and selective topics. Hematol Oncol Clin North Am. 2018;32(3):417–31.
Penas C, Navarro X. Epigenetic modifications associated to neuroinflammation and neuropathic pain after neural trauma. Front Cell Neurosci. 2018;12:158.
Garriga J, Laumet G, Chen SR, Zhang Y, Madzo J, Issa JPJ, Pan HL, Jelinek J. Nerve injury-induced chronic pain is associated with persistent DNA methylation reprogramming in dorsal root ganglion. J Neurosci. 2018;38:2616–7.
Liang L, Lutz BM, Bekker A, Tao YX. Epigenetic regulation of chronic pain. Epigenomics. 2015;7(2):235–45.
Retamal JS, Ramírez-García PD, Shenoy PA, Poole DP, Veldhuis NA. Internalized GPCRs as potential therapeutic targets for the management of pain. Front Mol Neurosci. 2019;12:273.
Laumet G, Chen SR, Pan HL. NMDA receptors and signaling in chronic neuropathic pain. In: Hashimoto K. (eds) The NMDA Receptors 2017. The Receptors, vol 30.
Pereira V, Goudet C. Emerging trends in pain modulation by metabotropic glutamate receptors. Front Mol Neurosci. 2019;11:464.
Machelska H, Celik MÖ. Recent advances in understanding neuropathic pain: glia, sex differences, and epigenetics. F1000Res. 2016;5:2743.
Calvo M, Davies AJ, Hébert HL, Weir GA, Chesler EJ, Finnerup NB, Levitt RC, Smith BH, Neely GG, Costigan M, Bennett DL. The genetics of neuropathic pain from model organisms to clinical application. Neuron. 2019;104(4):637–53.
Zorina-Lichtenwalter K, Parisien M, Diatchenko L. Genetic studies of human neuropathic pain conditions: a review. Pain. 2018;159(3):583–94.
Jones I, Yelhekar TD, Wiberg R, et al. Development and validation of an in vitro model system to study peripheral sensory neuron development and injury. Sci Rep. 2018;8:15961.
Wainger BJ, Buttermore ED, Oliveira JT, Mellin C, Lee S, Saber WA, Wang AJ, Ichida JK, Chiu IM, Barrett L, Huebner EA, Bilgin C, Tsujimoto N, Brenneis C, Kapur K, Rubin LL, Eggan K, Woolf CJ. Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat Neurosci. 2015;18(1):17–24.
Rayner MLD, Laranjeira S, Evans RE, Shipley RJ, Healy J, Phillips JB. Developing an in vitro model to screen drugs for nerve regeneration. Anat Rec (Hoboken). 2018;301(10):1628–37.
Nikolakopoulou P, Rauti R, Voulgaris D, Shlomy I, Maoz BM, Herland A. Recent progress in translational engineered in vitro models of the central nervous system. Brain. 2020;143(11):3181–213.
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–63.
Dyck P, Daube J, O’Brien P, Pineda A, Low P, Windebank A, et al. Plasma exchange in chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 1986;314:461–5.
Chang KL, Fillingim R, Hurley RW, Schmidt S. Chronic pain management: non-pharmacological therapies for chronic pain. FP Essent. 2015;432:21–6.
Shi Y, Wu W. Treatment of neuropathic pain using pulsed radiofrequency: a meta-analysis. Pain Phys. 2016;19(7):429–44.
Acknowledgements
The authors are grateful to the School of Life Sciences for providing necessary facilities for writing the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing of Interests
The authors declare no competing of interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human and animals subjects performed by any of the author.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All the authors have given the consent to publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collectiion on Nanotechnology, Nutraceuticals, and Immunotherapy in Cancer Research
Rights and permissions
About this article
Cite this article
Ameenudeen, S., Kashif, M., Banerjee, S. et al. Cellular and Molecular Machinery of Neuropathic Pain: an Emerging Insight. Curr Pharmacol Rep 8, 227–235 (2022). https://doi.org/10.1007/s40495-022-00294-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-022-00294-9