Abstract
Introduction
Sacituzumab govitecan (SG) is approved for patients with previously treated metastatic or locally advanced triple-negative breast cancer (TNBC), as per the ASCENT trial results. Real-world studies (RWSs) cover more diverse patients than clinical trials, offering crucial data for healthcare policies. This study aimed to investigate the safety and efficacy of SG in real-world Polish patients with previously treated metastatic TNBC.
Methods
In this ambispective multicenter cohort study, we collected demographic and clinical data. Premedication, adjustments in SG dosage, and treatment regimen adhered to the product's characteristics.
Results
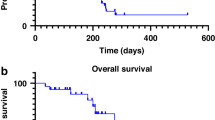
We included 79 female patients. The median age at SG initiation was 53 years; 32% of patients were initially diagnosed with a non-TNBC subtype. The median number of previous palliative lines was 2. Seven patients presented with brain metastases. The median overall survival was 10.3 months, and the median progression-free survival (PFS) was 4.4 months. The overall response rate was 35%, with a median time to response of 2 months. SG was discontinued by 70% of patients, primarily due to disease progression (95%). Treatment delays due to adverse events (AEs) occurred in 67% and dose reductions in 25% of patients, with neutropenia being the most common. Grade ≥ 2 AEs included neutropenia (43%), anemia (10.1%), and diarrhea (4%). A longer interval between breast cancer diagnosis and SG initiation or between metastasis diagnosis and SG initiation correlated with improved PFS, likely reflecting the disease's biological aggressiveness rather than treatment efficacy.
Conclusion
In this RWS, SG demonstrated effectiveness and safety in patients with previously treated metastatic TNBC, consistent with ASCENT trial outcomes. Further research is needed to explore the efficacy of SG in different patient populations and healthcare systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Real-world studies offer valuable insights into the effectiveness and safety of treatments in everyday clinical settings. |
This study includes patients with previously treated metastatic triple-negative breast cancer (TNBC) from five reference Polish cancer centers, representing one of the largest real-world studies to date, with 79 patients treated with sacituzumab govitecan (SG). |
SG demonstrated notable clinical benefit for patients with previously treated metastatic TNBC, with median overall survival of 10.3 months and progression-free survival of 4.4 months. |
The results support the efficacy and tolerable toxicity profile of SG observed in the ASCENT trial, confirming its antitumor effectiveness in a real-world setting. |
Introduction
The prognosis for patients with advanced breast cancer (BC) is significantly influenced by the specific subtype of BC they have. Gene expression analyses have classified BC into five biological subtypes: luminal A, luminal B, basal, "normal-like," and human epidermal growth factor receptor 2 (HER2)-positive BC. In clinical settings, these classifications are substituted with equivalents derived from routinely evaluated pathomorphological characteristics of invasive BC, including estrogen receptor (ER) status, progesterone receptor (PgR) status, the Ki-67 proliferation index, and HER2 status [1, 2]. Triple-negative breast cancer (TNBC) is characterized by the absence of ER/PgR and negative HER2 status [1, 2].
Metastatic TNBC treatment varies based on programmed death-ligand 1 (PD-L1) expression and breast cancer gene (BRCA) mutation status. For BRCA wild-type patients with a PD-L1 combined positive score (CPS) of ≥ 10, pembrolizumab-based chemo-immunotherapy is preferred [3,4,5]. In other cases, single or combination chemotherapy is used, with the regimen choice influenced by prior exposure to cytotoxic agents, comorbidities, and previous response duration. Common options include anthracyclines and taxanes, with monotherapy often favored for better tolerance [3,4,5]. For patients with BRCA mutation, poly (ADP-ribose) polymerase (PARP) inhibitors are recommended unless the patient is chemotherapy-naïve or has progressed on PARP therapy, in which case platinum-based treatments are preferred [3, 4] Subsequent options include sacituzumab govitecan (SG) or trastuzumab deruxtecan (T-DXd) for HER2-low tumors and pembrolizumab for tumors with high tumor mutational burden and microsatellite instability–high/deficient mismatch repair profiles [3, 4].
SG is an antibody‒drug conjugate (ADC) that combines a monoclonal antibody directed against trophoblast cell surface antigen 2 (TROP-2) with SN-38, the active metabolite of irinotecan, linked via a hydrolyzable linker [6, 7]. TROP-2 is a transmembrane calcium signal transducer that is overexpressed in several cancers, including BC. In BC, higher TROP-2 expression is correlated with poorer survival [7, 8]. In 2020, the Food and Drug Administration (FDA) granted accelerated approval for SG for the treatment of patients with metastatic TNBC who had received at least two prior lines of therapy. This approval was supported by data from the IMMU-132–01 phase I/II trial, which demonstrated efficacy in 108 patients with metastatic TNBC [9]. Following the results of the ASCENT trial, the approval was converted to a regular one. In this phase III trial, SG was administered after at least two lines of systemic therapy, including one for metastatic disease and one with a taxane-based regimen. The ASCENT trial reported significant improvements in overall survival (OS) and progression-free survival (PFS) compared to standard treatment [6]. Recent final results from ASCENT confirm the OS and PFS benefits and indicate a manageable toxicity profile [10].
Real-world studies (RWSs) are essential because they offer valuable insights into the effectiveness and safety of treatments in everyday clinical settings, encompassing a broader and more diverse patient population than those typically included in controlled clinical trials. By filling the gaps left by clinical trials, these studies provide crucial data that can guide more applicable healthcare policies and decision-making [11, 12]. The limited data regarding RWSs that explore SG in the TNBC setting are presented in Table 1 [13,14,15,16,17,18].
The aim of this cohort study was to assess the real-world efficacy and safety of SG in patients with metastatic TNBC treated within five Polish oncology centers.
Methods
Study Participants and Data Collection
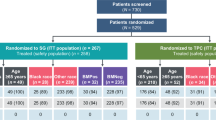
Patients with locally advanced or metastatic TNBC who received SG as part of routine clinical care within a national reimbursement program at five Polish oncology centers were identified and included in this study. SG treatment was initiated between November 2022 and April 2024. Additionally, patients who received treatment during early reimbursement programs (outside of clinical trials) were allowed to participate in the study. There were no limitations regarding patient sex. Patients receiving SG treatment within a clinical trial were excluded.
Patient eligibility for SG administration under the Polish national reimbursement program has been included in the Supplementary Materials. For patients needing dose adjustments, the strategy followed the European Union’s product characteristics information. This involved a stepwise dosage reduction from the standard 10.0 mg/kg to an initial lower dose of 7.5 mg/kg and further reduction to 5.0 mg/kg as necessary [19, 20].
Patients were identified through hospital registry systems. The following data regarding disease diagnosis, treatment efficacy and safety, and patient sociodemographic characteristics were collected retrospectively directly from patient medical records: age, sex, and detailed clinical information: primary tumor location and sites of distant metastatic disease. The following data were collected prospectively: the timeline and doses of systemic treatments administered and data related to imaging studies and response to the treatment. These data were recorded based on standardized treatment protocols and imaging criteria outlined in the patient management documentation. Additionally, data on the initial treatment objective (whether palliative or radical) at the time of diagnosis, current survival status, and dates of the most recent follow-up were collected from patient medical records. Histopathological characteristics, including tumor histology; the status of ER, PgR, HER2, and Ki-67 in the latest and initial specimens; and tumor grade, were also documented using pathology reports from patient files.
Study Objectives
The primary objective of this study was to assess PFS and OS in a real-world population of patients with TNBC treated with SG. The secondary objective was to assess the toxicity profile of this treatment.
PFS was measured from the date of initiation of SG treatment to the earliest occurrence of either disease progression or death. OS was calculated from the first dose of SG to the date of death from any cause. Adverse events (AEs) were categorized in alignment with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 5.0. Radiological responses were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The objective tumor response rate (ORR) was assessed as the percentage of patients in a study who achieved a PR or CR as the best response rate.
Ethical Considerations
This study was approved by the ethical committees of the Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw Branch, Poland (reference number 21/2024, dated 22 February 2024) and Krakow Branch (reference number 2/2023 dated 18 April 2023). All procedures involving human participants in this study adhered to the ethical standards of the institutional ethical committee and the principles outlined in the 1964 Helsinki Declaration, along with its subsequent amendments or equivalent ethical guidelines. Informed consent and standard institutional informed consent were obtained from each patient prior to initiating treatment with SG under the reimbursement national program. Informed consent for retrospective data gathering was not required as determined by the ethical committee.
All procedures followed applicable guidelines and regulations.
Statistical Analysis
The means, standard deviations, medians, quartiles, and ranges of quantitative variables are shown. For qualitative variables, absolute and relative frequencies (N and %) are reported. Univariate Cox regression (proportional hazards model) was employed to model the potential impact of predictors on OS and PFS. Hazard ratios (HRs), alongside the 95% confidence intervals, are presented. The significance level was set to 0.05. All the analyses were conducted in R software, version 4.4.0.
Results
Patient Characteristics
This study analyzed a cohort of 79 patients diagnosed with metastatic TNBC. All patients were female and of European descent. At the time of SG initiation, the median age was 53 years (interquartile range, 44–64 years), and the mean age was 54 years, with a standard deviation of 12.7 years (range, 31–77 years). The median Ki-67 index was 50% (interquartile range, 35–78%), with a mean of 55% ± 25% (range, 5–100%; n = 75). Thirty-two percent of patients were initially diagnosed with the non-TNBC subtype. Additional clinicopathological data for the patients can be found in Table 2. All participants had distant metastases at the commencement of the study.
The median follow-up was 5.9 months (range 0.8–30.9). The median time from initial BC diagnosis or metastatic BC diagnosis to SG initiation was 3.0 years and 8.6 months, respectively.
The data regarding previously received (neo)adjuvant systemic treatment (N = 67) and palliative systemic treatment (all participants, N = 79) as well as AEs reported prior to SG initiation are presented in Table 3.
The median number of palliative treatment lines before SG was 2 (range, 1–5).
Treatment Characteristics
Overall, 79 patients started SG treatment. Among them, 55 (69.6%) discontinued the treatment. The most common cause of SG discontinuation was disease progression (N = 52 patients, 94.5% of patients who stopped the treatment). The median number of full SG cycles for those who discontinued treatment was 4, with a mean of 5.8 ± 5.4 (range 1–21). In patients with a known radiological response according to the RECIST 1.1 criteria (n = 68), the ORR was 35.3%. The median time to achieve a response was 2 months (range 0.7–7.6 months, N = 61). Table 4 presents the radiological response and treatment outcome data. The median number palliative treatment lines for SG was 3 (2–6), with 39 patients (49.4%) receiving SG on the second and 40 patients (50.6%) on the third line and beyond.
Thirty-seven patients who progressed to SG started subsequent systemic treatment (67.3%).
Treatment Outcomes and Influencing Factors
The median OS was 10.3 months (range 0.8–30.9). The median PFS was 4.4 months (range 0.7–16.1). Table 5 and Fig. 1 present the OS and PFS data. For patients without brain metastases, the OS was 10.4 months (range 0.8–30.9). The median PFS was identical to that of the unselected population.
According to the univariate Cox proportional hazards model, each additional year between BC diagnosis and the initiation of SG treatment reduced the probability of progression or death at any given time by 12.8% (HR = 0.872), with a p value of 0.021. Each additional month between the diagnosis of metastases and the commencement of SG treatment decreased the likelihood of progression or death at any given time by 2.6% (HR = 0.974), with a p value of 0.009.
Each additional year between cancer diagnosis and the initiation of SG therapy reduced the risk of death at any point in time by 16.2% (HR = 0.838; p = 0.031). The use of pembrolizumab in palliative care in an earlier line of systemic treatment increased the likelihood of death at any time by 4.498 times (HR = 4.498), with a p value of 0.042. A treatment-related decreased platelet count in patient history before initiating SG therapy increased the risk of death at any given time by 2.831 times (HR = 2.831, p = 0.016), and a decreased platelet count following SG therapy increased the risk of death at any point in time by 2.859 times (HR = 2.859), with a p value of 0.017. No other factors (Tables 2, 3, and 4) were related to OS or PFS, including treatment delay, SG dose reduction, SG treatment line (2nd vs. 3rd+), or type of radiological response to SG treatment (ORR vs SD).
Treatment Safety
Of the 55 patients who stopped treatment with SG, only two discontinued due to AEs, and one due to unknown cause. Taking into account the whole population, in 53 patients (67.1%) treatment was delayed at some point due to AEs, with a median time to first delay of 22 days (range 8–169 days). The most common cause of first treatment delay was neutropenia (N = 40, 50.6%). Twenty patients (25.3%) required dose reduction, with a median time to first dose reduction of 29 days (range 1–232 days). The most common cause for dose reduction was also neutropenia (N = 13, 16.5%). AEs > grade 2 reported during treatment with SG are presented in Table 6.
Alopecia (any grade) was reported in 27 patients (34.2%).
Discussion
SG has garnered attention for its manageable safety profile and promising survival outcomes in patients with metastatic TNBC refractory to prior lines of palliative chemotherapy. Drawing from a multicenter national study in Poland, we aimed to explore the safety and efficacy of SG in a real-world population.
Our patient cohort resembled the ASCENT trial population in terms of median age, distribution of visceral metastases to the lungs and liver, percentage of patients with HER2-low TNBC, number of previous anticancer regimens (including lines in the [neo]adjuvant setting), and previous application of taxanes. In our cohort, all patients were white, whereas in the ASCENT trial, 20% of the population was reported as belonging to other racial or ethnic groups. Additionally, the rates of metastasis to lymph nodes and bones were greater in our real-world data. The time from initial diagnosis to diagnosis of metastatic disease was almost twice as long in the ASCENT trial as in our cohort [6]. This interval was shown to have a positive impact on PFS in our study: a longer interval between BC diagnosis and SG initiation and between diagnosis of metastases and SG initiation was linked to improved PFS. This outcome likely reflects the inherent biological aggressiveness of the disease rather than a stronger therapeutic response. Patients with TNBC who experience an early relapse following (neo)adjuvant chemotherapy typically exhibit a more aggressive disease course [21]. In the ASCENT trial, 14% of patients received a single line of therapy in the metastatic setting and had recurrence of disease within 12 months of (neo)adjuvant chemotherapy. SG extended PFS and OS with a manageable safety profile. That was consistent with the overall study population [21]. In our cohort, nearly 50% of patients received SG as a second-line treatment.
Age was not shown in our study to influence the treatment outcome, confirming earlier results [6, 22]. Approximately 30% of the patients in the registration phase III study were not initially diagnosed with TNBC, which was comparable to our study (32%) [6, 23]. Similar or even higher percentages have been registered in other RWSs [14, 16]. This inconsistency between the staining of the primary tumor and that of the metastatic site is a well-known phenomenon [24, 25] and emphasizes the importance of obtaining biopsy samples at the time of metastasis or recurrence. As shown by analysis of patients in the ASCENT study, patients without TNBC at initial diagnosis had better outcomes and a manageable safety profile with SG, supporting its effectiveness regardless of initial subtype [23].
In our cohort, we showed median PFS of 4.4 months and median OS of 10.3 months. These outcomes are slightly lower than those reported in the ASCENT trial, which showed median PFS of 5.6 months and OS of 12.1 months for patients without brain metastases. When including patients with brain metastases, the ASCENT trial reported median PFS of 4.8 months and OS of 11.8 months [6]. Our findings align with other RWSs, which have consistently reported median PFS of 3–5 months and median OS ranging from 8 to 21 months [13,14,15,16,17,18, 26]. This consistency supports the efficacy of SG in treating patients with metastatic TNBC, despite variations in patient populations.
In the ASCENT trial, the benefit of SG was observed across all clinical and prespecified subgroups, including patients who had previously been treated with immune checkpoint inhibitors (ICIs), PD-1 inhibitors, or PD-L1 inhibitors (27% of the population). Although the impact of first-line ICIs on the efficacy of SG in subsequent lines has not been explored, we consider this an interesting topic. This group of patients had OS and PFS of 4.2 months, which is numerically lower than the data obtained in the general population. Our study suggested worse outcomes in these patients in terms of OS; however, the group was very small (see Table 2), and only univariate analysis was applied. Further RWSs could provide more evidence on this topic.
Our data do not confirm poorer OS in patients with central nervous system involvement. In a small study by Singh et al., PFS for patients without and with brain metastases was 4.4 months and 6.0 months, respectively, with identical OS [17]. In other RWSs, these patients present with numerically worse outcomes [14]. According to the ASCENT trial results, the whole population (with and without brain metastases) presented with better outcomes in the SG group than in the standard therapy group [6].
The American Society of Clinical Oncology (ASCO) data presented by Kalinsky et al. revealed differences in OS between patients treated with palliative treatment via the second line or third line and beyond (13.9 vs. 8.4 months) [18]. Although we did not observe such differences in our study, this aspect should be reported in other RWSs.
In our patients with a known radiological response, the ORR was 35%, mirroring data from clinical trials [6] and other RWSs [13, 14]. The median time to achieving a response was 2 months in our study, which was slightly longer than the 1.5 months reported in the ASCENT trial, likely due to differences in the timing of the first radiological assessment [6].
In our study, dose reduction was necessary for 25.3% of patients, primarily due to neutropenia. In other RWSs, 17–54% of patients required dose modifications [13,14,15,16], while in the ASCENT trial, this percentage was 22% [6]. Only 2.5% of patients in our cohort discontinued treatment due to AEs, and this percentage is consistent with other scarce available data (1–7%) [13,14,15,16] and registration trials [6].
There are significant differences in reporting safety data in published RWSs. Not only do the results differ in the frequency of different AEs reported, but the method of reporting these data also varies [13,14,15,16]. Our data regarding the most frequent treatment-related AEs of grade > 2 were consistent with clinical trial results and RWS, including neutropenia in 43% of patients (51% in the ASCENT trial [6], approximately 30% in the RWS trial) [13, 14, 16]) and anemia in 10% (8% in the ASCENT trial). Similarly, a study published by Caputo et al. reported a numerically lower incidence of diarrhea (4%) [13] (10% in the ASCENT trial and as high as 15–19% in some other RWSs)[15, 16]) and febrile neutropenia (3%, 6% in the ASCENT trial) [6]. We also noted a 5% incidence of grade 3 or higher platelet count decrease.
Alopecia of any grade was reported in 34% of patients. There is a wide range in the incidence of this complication: 46% in the ASCENT trial, 91% reported by Reinisch et al., and 67% by Caputo et al. [6, 13, 16]. Some RWSs did not report on this AE [14, 15]. It is possible that the reporting of this AE, which is not treatment-limiting toxicity, could be underrepresented in our cohort.
Study Limitations
This study is subject to various constraints that warrant attention. First, the relatively small sample size restricts the depth of subgroup analyses and precludes adjustments based on important patient characteristics. Second, its partially retrospective design hinders the establishment of causal relationships between variables. These are typical limitations of RWSs.
Moreover, the absence of a comparative group prohibits direct comparisons with standard chemotherapy and impedes the assessment of the relative efficacy of SG. Additionally, the lack of data on previous medication, including granulocyte colony-stimulating factor (G-CSF) application, underscores a notable gap in our understanding of its impact on treatment outcomes. We plan to gather these data to explore its influence on treatment outcomes and safety issues. We have not assessed TROP-2 expression in our population, so we cannot elaborate on the relationship between TROP-2 expression and SG efficacy.
Data regarding PD-L1 status were not collected. This is because the majority of patients received their first-line treatment for TNBC before the reimbursement of chemo-immunotherapy with pembrolizumab in Poland, and therefore, CPS assessment was not conducted. In addition, chemo-immunotherapy with atezolizumab has never been reimbursed in Poland.
Furthermore, the study's limited scope, confined to investigational sites in Poland, raises concerns about the generalizability of the findings to broader populations and other healthcare settings. These limitations highlight the need for further research. Addressing these gaps through larger-scale studies, comprehensive data collection, and meticulous control of confounders will provide a more nuanced understanding of the real-world effectiveness and safety profile of SG.
Conclusions
SG is currently endorsed by major guidelines for the treatment of patients with metastatic TNBC who have undergone at least two prior therapies, with at least one being in the metastatic setting [3, 4].
Our RWS on the use of SG in the Polish population demonstrates that its efficacy aligns closely with the findings from the ASCENT clinical trial. The manageable safety profile and acceptable survival outcomes observed in our study support the integration of SG into routine clinical practice for the treatment of patients with metastatic TNBC. Despite the limitations inherent to RWS studies, our findings provide valuable insights into the practical application of SG in a real-world setting. The observed AEs were consistent with those reported in clinical trials and other RWSs, with neutropenia being the most common cause of treatment delays and dose reductions. These results underscore the importance of continued post-approval surveillance and data collection to optimize patient outcomes and refine the management of adverse effects associated with SG therapy. The RWS also raises new clinically important questions that have not been addressed in clinical trials, such as the efficacy of SG after the first-line application of anti-PD-1 inhibitors.
Data Availability
Interested parties can obtain access to the data by contacting the corresponding author upon request.
References
Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol. 2014;5(3):412–24. Available from: https://pubmed.ncbi.nlm.nih.gov/25114856/. Accessed 29 Mar 2024.
Rakha EA, Tse GM, Quinn CM. An update on the pathological classification of breast cancer. Vol. 82, Histopathology. John Wiley and Sons Inc; 2023. p. 5–16.
Triple-negative Breast Cancer | ESMO https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/triple-negative-breast-cancer. Available from: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/triple-negative-breast-cancer. Accessed 28 May 2024
Rashmi Kumar N, Schonfeld R, Gradishar WJ, Lurie RH, Moran MS, Abraham J, et al. NCCN Guidelines Version 2.2024 Breast Cancer. 2024. Available from: https://www.nccn.org. Accessed 3 Apr 2024.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28. Available from: https://pubmed.ncbi.nlm.nih.gov/33278935/. Accessed 23 Mar 2023.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021;384(16):1529–41. Available from: https://pubmed.ncbi.nlm.nih.gov/33882206/. Accessed 23 Mar 2024.
Püsküllüoğlu M, Rudzińska A, Pacholczak-Madej R. Antibody-drug conjugates in HER-2 negative breast cancers with poor prognosis. Biochim Biophys Acta Rev Cancer. 2023;1878(6). Available from: https://pubmed.ncbi.nlm.nih.gov/37758021/. Accessed 17 Oct 2023.
Shastry M, Jacob S, Rugo HS, Hamilton E. Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer. Breast. 2022;66(October):169–77.
Wahby S, Fashoyin-Aje L, Osgood CL, Cheng J, Fiero MH, Zhang L, et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin Cancer Res. 2021;27(7):1850–4. Available from: https://pubmed.ncbi.nlm.nih.gov/33168656/. Accessed 16 Aug 2024.
Bardia A, Rugo HS, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J Clin Oncol. 2024;42(15):1738–44. Available from: https://pubmed.ncbi.nlm.nih.gov/38422473/. Accessed 9 Jun 2024.
Booth CM, Karim S, Mackillop WJ. Real-world data: towards achieving the achievable in cancer care. Nat Rev Clin Oncol. 2019;16(5):312–25. Available from: https://pubmed.ncbi.nlm.nih.gov/30700859/. Accessed 10 Jun 2024.
Tang M, Pearson SA, Simes RJ, Chua BH. Harnessing Real-World Evidence to Advance Cancer Research. Current Oncology. 2023;30(2):1844.
Caputo R, Buono G, Piezzo M, Martinelli C, Cianniello D, Rizzo A, et al. Sacituzumab Govitecan for the treatment of advanced triple negative breast cancer patients: a multi-center real-world analysis. Front Oncol. 2024;14. Available from: https://pubmed.ncbi.nlm.nih.gov/38595817/. Accessed 10 Jun 2024.
De Moura A, Loirat D, Vaillant S, Korbi S, Kiavue N, Bello Roufai D, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer patients treated at Institut Curie Hospitals: efficacy, safety, and impact of brain metastases. Breast Cancer. 2024. Available from: https://pubmed.ncbi.nlm.nih.gov/38600429/. Accessed 10 Jun 2024.
Hanna D, Merrick S, Ghose A, Devlin MJ, Yang DD, Phillips E, et al. Real world study of sacituzumab govitecan in metastatic triple-negative breast cancer in the United Kingdom. British J. Cancer 2024. 2024;1–5. Available from: https://www.nature.com/articles/s41416-024-02685-9. Accessed 10 Jun 2024.
Reinisch M, Bruzas S, Spoenlein J, Shenoy S, Traut A, Harrach H, et al. Safety and effectiveness of sacituzumab govitecan in patients with metastatic triple-negative breast cancer in real-world settings: first observations from an interdisciplinary breast cancer centre in Germany. Ther Adv Med Oncol. 2023;15. Available from: https://pubmed.ncbi.nlm.nih.gov/37789989/. Accessed 10 Jun 2024.
Singh V, Dhaibar H, Peddi P. Real-world outcomes of sacituzumab govitecan in breast cancer. 2024;42(16_suppl):e13137. https://doi.org/10.1200/JCO.2024.42.16_suppl.e13137
Kalinsky K, Spring L, Yam C, Taylor A, Sjekloca N, Kaushiva A, et al. Real-world outcomes in patients (pts) with metastatic triple-negative breast cancer (mTNBC) treated with sacituzumab govitecan (SG) in 2L+ in the United States (US). 2023;41(16_suppl):e18879–e18879. https://doi.org/10.1200/JCO.2023.41.16_suppl.e18879. Accessed 10 Jun 2024.
TRODELVY® (sacituzumab govitecan-hziy). US Food and Drug Administration www.fda.gov. Available from: www.fda.gov/medwatch. Accessed 28 Mar 2023.
Obwieszczenia ministra zdrowia - lista leków refundowanych. Available from: https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych. Accessed 17 May 2024.
Carey LA, Loirat D, Punie K, Bardia A, Diéras V, Dalenc F, et al. Sacituzumab govitecan as second-line treatment for metastatic triple-negative breast cancer—phase 3 ASCENT study subanalysis. npj Breast Cancer. 2022;8(1):1–7. Available from: https://www.nature.com/articles/s41523-022-00439-5. Accessed 16 Aug 2024.
Hurvitz SA, Bardia A, Punie K, Kalinsky K, Carey LA, Rugo HS, et al. Subgroup analyses from the phase 3 ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. npj Breast Cancer 2024;10:1–11. Available from: https://www.nature.com/articles/s41523-024-00635-5. Accessed 16 Aug 2024.
O’Shaughnessy J, Brufsky A, Rugo HS, Tolaney SM, Punie K, Sardesai S, et al. Analysis of patients without and with an initial triple-negative breast cancer diagnosis in the phase 3 randomized ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Breast Cancer Res Treat. 2022;195(2):127–39. https://doi.org/10.1007/s10549-022-06602-7.
Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15(11):1164 (/pmc/articles/PMC3227913/).
Aktas B, Kasimir-Bauer S, Müller V, Janni W, Fehm T, Wallwiener D, et al. Comparison of the HER2, estrogen and progesterone receptor expression profile of primary tumor, metastases and circulating tumor cells in metastatic breast cancer patients. BMC Cancer. 2016;16(1). Available from: https://pubmed.ncbi.nlm.nih.gov/27456970/. Accessed 12 Jun 2024.
Walsh EM, Klar M, Abuhadra N, Robson M, Drago J. 419P Aspire to ASCENT: Real-world outcomes from patients with metastatic triple-negative breast cancer (mTNBC) treated with Sacituzumab govitecan (Saci) in a single academic institution. Ann. Oncol. 2023;34:S359. Available from: http://www.annalsofoncology.org/article/S0923753423014321/fulltext. Accessed 12 Jun 2024.
Funding
No financial support was provided for the preparation of this manuscript. No funding or sponsorship was received for publication of this article.
Author information
Authors and Affiliations
Contributions
Miroslawa Püsküllüoğlu collectively designed and conceptualized the research. All authors were actively involved in organizing the database or collecting patient data. Miroslawa Püsküllüoğlu led all the statistical analyses. Ethical approval was obtained through the collaborative efforts of Miroslawa Püsküllüoğlu, Aleksandra Grela-Wojewoda, Joanna Streb, and Marek Ziobro. Miroslawa Püsküllüoğlu wrote the initial draft of the manuscript, with specific contributions from Renata Pacholczak-Madej and Agnieszka Rudzińska. All the authors, including Małgorzata Pieniążek, Manuela Las-Jankowska, Paulina Kilian-Van Miegem, Aleksandra Łacko, Michał Jarząb, and Anna Polakiewicz-Gilowska actively participated in revising or commenting the manuscript. The final version was approved by all the authors.
Corresponding author
Ethics declarations
Conflict of Interest
Miroslawa Püsküllüoğlu obtained travel grants and lecture honoraria from AstraZeneca, Roche, Novartis, Elli Lilly, Janssen, Gilead and Amgen; Małgorzata Pieniążek travel grants and lecture fees from Pfizer and Novartis; lecture fees from Gilead, advisory board from Novartis; Manuela Las-Jankowska, Joanna Streb and Paulina Kilian-Van Miegem report no conflicts of interest. Marek Ziobro reports travel grants and lecture honoraria from Pierre Fabre, Novartis, Ipsen; Renata Pacholczak-Madej travel grants from Accord, GSK, BMS, lecture fees from BMS; Agnieszka Rudzińska from Gilead, BMS, Sandoz; Aleksandra Grela-Wojewoda from Novartis, BMS, Pierre Fabre, Roche, Amgen, MSD, Gilead and Pfizer, Aleksandra Łacko from Astra Zeneca, Pfizer, Novartis, Eli Lilly, Roche, Gilead Science including advisory board member role; Michał Jarząb conference fees by Gilead, Roche, speaker’s honoraria by Novartis, Roche, Lilly, Pfizer, Teva, Exact Sciences, Mammotome, advisory boards by Novartis, Pfizer; and Anna Polakiewicz-Gilowska lecture honoraria and travel grants from AstraZeneca, Roche, Novartis, Elli Lilly, Swixx, Gilead and Pfizer.
Ethical Approval
All activities conducted in studies involving human participants adhered to the ethical guidelines set forth by the institutional ethical committee and were in accordance with the principles outlined in the 1964 Helsinki Declaration and its subsequent amendments or equivalent ethical standards. This study was approved by the Ethical Committees of the Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw Branch, Poland (reference number 21/2024, dated 22 Feb 2024) and Krakow Branch (reference number 2/2023 dated 18 April 2023). Informed consent, including standard institutional consent, was obtained from each patient before initiating SG treatment under the national reimbursement program. The Ethical Committee determined that informed consent for retrospective data collection was not required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Püsküllüoğlu, M., Pieniążek, M., Las-Jankowska, M. et al. Sacituzumab Govitecan for Second and Subsequent Line Palliative Treatment of Patients with Triple-Negative Breast Cancer: A Polish Real-World Multicenter Cohort Study. Oncol Ther (2024). https://doi.org/10.1007/s40487-024-00307-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40487-024-00307-1