Abstract
The gut microbiota plays a crucial role in maintaining homeostasis in the human gastrointestinal tract. Numerous studies have shown a strong association between the gut microbiota and the emergence and progression of various diseases. Leukemia is one of the most common hematologic malignancies. Although standardized protocols and expert consensus have been developed for routine diagnosis and treatment, limitations remain due to individual differences. Nevertheless, a large number of studies have established a link between the gut microbiota and leukemia, with disturbances in the gut microbiota directly or indirectly affecting the development of leukemia. However, the causal relationship between the two remains unclear, and studying and exploring the causal relationship may open up entirely new avenues and protocols for use in the prevention and/or treatment of leukemia, offering new insights into diagnosis and treatment. In this review, the intricate relationship between the gut microbiota and leukemia is explored in depth, including causal associations, metabolite effects, therapeutic applications, and complications. Based on the characteristics of the gut microbiota, the future applications and prospects of gut microbiota are discussed to provide useful information for clinical treatment of leukemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study ? |
Conventional treatments for leukemia have limitations. The gut microbiota is closely associated with multiple diseases, but its relationship with leukemia is unclear, and there is a need for research. |
This study was carried out to explore the relationship between the gut microbiota and leukemia, and how to utilize this relationship to provide new strategies for the treatment and management of leukemia. |
What was learned from the study? |
There is a close connection between the gut microbiota and the occurrence, development, treatment effect, and complications of leukemia. |
Even if some results are inconsistent with the initial hypothesis, they provide new directions and ideas for future research. Whether the results are negative, neutral, or affirmative, they all contribute to further optimizing the research and treatment strategies for leukemia. For example, a deeper exploration of the mechanism of the gut microbiota in leukemia treatment can promote the development of personalized treatment regimens. |
Introduction
Acute leukemia (AL) is a common blood cancer and is characterized by a malignant tumor of the hematopoietic system. According to the literature, the malignant features of leukemia include uncontrolled proliferation of leukemic cells, invasion of normal tissues and organs, and the possibility of metastasis, which is often assessed by various aspects such as cell morphology and genetic characteristics [1,2,3]. According to Global Cancer Statistics 2020, leukemia has a mortality rate of 3.1% [4]. Among youth and young adults, highly malignant acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) are two common types of leukemia [5]. Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in Western countries [6, 7], and chronic myeloid leukemia (CML), a myeloproliferative neoplasm caused by the fusion gene BCR:ABL1, accounts for 15% of all newly diagnosed adult leukemia cases [8, 9]. A comprehensive set of international standardized expert consensus guidelines has been established for the routine management of leukemia [2, 10]. However, given the complexity, heterogeneity, and individual variability of different types of leukemia, the therapeutic effects remain limited to some extent. Therefore, it is important to further investigate the pathogenesis of leukemia and to explore new treatment strategies.
Within the larger community of microorganisms, the relationship between the gut microbiota and leukemia has long been established. The homeostasis of the gut microbiota is influenced by a variety of factors, both genetic and environmental [11]. It begins to colonize the neonate gut after birth and remains present throughout life [12,13,14,15]. However, disturbances in the gut microbiota directly or indirectly affect both healthy and diseased individuals [16,17,18,19]. In addition to leukemia, they has been associated with a variety of diseases [20,21,22,23], including respiratory diseases, central nervous system diseases, and tumors. Studies focusing on the gut microbiota to explore causal relationships with different types of leukemia may open up new avenues and options for the diagnosis and treatment of the disease. Emerging lines of inquiry may focus on the development of potential new strategies for the prevention and/or treatment of leukemia. This review focuses on recent studies of the gut microbiota and leukemia to explore the causal relationship and provide potential options for clinical treatment.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Gut Microflora and Leukemia Causality
The intestinal microbiota, as a complex microbial system, consists of many species of microbial communities that exhibit certain similar characteristics. Among them, the genus Anabaena and phylum Actinobacteria are overwhelmingly dominant in terms of numbers [24, 25]. The number of microorganisms in the leukemia community is very high [18, 19]. However, different types of leukemia may also affect the composition of the intestinal flora [26, 27] (Table 1). For example, Streptococcus spp. are most common in leukemia, while the families Lachnospiraceae and Ruminococcaceae are less common [28]. The gut microbiota is involved in the development and maturation of the immune system and in the regulation of immune responses and pathogen resistance, among other functions [29]. It was reported that the short-chain fatty acids (SCFAs) secreted by Escherichia coli obtained from healthy populations effectively inhibited the proliferation of tumor cells and exerted anti-cancer and anti-inflammatory effects [30]. In addition to protective mechanisms, analysis of macrogenomic samples from patients with different cancers has shown that the gut microbiota can be used as a diagnostic bacterial marker for a variety of diseases [31].
In order to investigate the causal association between gut microbiota and leukemia, in recent years a large number of extensive studies have examined the gut microbiota in children with ALL, including the application of survival prediction based on the variability in gut microbiota, and the assessment of the risk of infection during chemotherapy for childhood ALL [32,33,34,35]. This article attempts to summarize some of the studies on gut microbiota and childhood ALL (Table 2). Studies have shown that before the treatment of ALL in children, variations in their intestinal microflora are observed, and bacteria with protective mechanisms, such as Enterococcus faecalis and Bacteroides, show a decrease or even absence, while the overproliferation of enterococci exacerbates intestinal damage and inflammatory response. Such specific changes are strongly associated with the course of childhood ALL. In the case of ALL, some studies have used mice to construct an animal model of pregenetic B-cell ALL (pB-ALL), and the results have shown that the lack of a commensal microbiota, rather than a characteristic microbiota, promotes leukemia development [36]. Differential microbiota colonization may also be associated with infant leukemia development, as reported by the Children's Oncology Group [37]. These findings confirm the interaction between the gut microbiota and AL occurrence and development. However, the relevant literature shows inconsistent results for alterations in specific microbiota genera [28, 38]. For example, the relative abundance of Edwardsiella tarda and Prevotella maculosa was positively correlated with interleukin 10 levels [39,40,41]. This reveals the inconsistency in recent studies in terms of the depth of sequencing of gut microorganisms and issues with small sample sizes of individual studies; thus, more standardized, large-scale deep sequencing samples are needed for future analyses.
In patients with CLL, it was found that the gut microbiota was most enriched in species of Bacteroidaceae, Clostridiaceae, Prevotellaceae, and Actinetobacteraceae species, whereas those of the families Lachnospiraceae and Ruminococcaceae exhibited depletion [42]. Compared to healthy individuals, patients with CLL have reduced diversity and variability of bacterial phyla of the gut microbiota, as evidenced by an increased abundance of thick-walled phyla and a decreased abundance of Anaplasma [43].
Previous studies have shown that the gut flora of AML is disrupted during induction chemotherapy (IC). The baseline diversity of the gut microbiome has been associated with the risk of infection during IC in AML, and assessing the diversity of the gut microbiota during IC may reduce the complications of AML infections [44, 45]. Interestingly, after cessation of chemotherapy, the microbiota produced new communities that were highly distinct from baseline and showed persistent alterations, whereas the abundance of Bacteroides immitis remained low [46]. Pötgens et al. reported that intensive treatment of AML temporarily impaired gut barrier function and induced long-lasting changes in the composition and metabolic activity of the gut microbiota, which correlate with the characteristics of cachexia [47].
It has been shown that Actinobacteria, Acidobacteria, and Chloroflexota are present in high abundance in the gut microbiota of patients with CML, while Mycoplasmatota (formerly Tenericutes) had relatively low abundance. Yu et al. performed a comparison study of the gut microbiota of patients with myeloid leukemia (ML) and controls using 16S rRNA analysis and found that Streptococcus spp. exhibited increased abundance in the microbiome of both patients with AML and CML [48]. However, lactic acid, as a major compound produced by Streptococci during sugar fermentation, may have an impact on the progression of CML [49]. An increase in streptococcal abundance might have a detrimental effect on leukemia; however, Actinomycetes abundance could potentially help to reduce adverse reactions. Studies have shown that bacteria of the phylum Actinobacteria may be beneficial for patients with AL, exhibiting antioxidant activity [50, 51].
In children with ALL, diversity and variability in the gut microbiota prior to treatment correlate with disease duration, and factors such as the lack of a commensal microbiota promote progression, whereas in patients with CLL, decreased diversity and variability is found in the bacterial phyla of the gut microbiota. In patients with AML, the gut flora is disrupted during chemotherapy, and intensive treatment impairs gut function and leads to lasting changes. In patients with CML, changes are found in the abundance of some of the bacterial phyla of the gut microbiota, as evidenced by changes in the abundance of Streptococcus and Actinomyces. To date, studies on gut microbiota and cancer have shown a causal relationship between them [52].
Gut Microbiota and Other Diseases
In addition to leukemia, we have identified associations between the gut microbiota and several other diseases, both oncological and non-oncological. Seventeen strong associations have been demonstrated between genetic susceptibility of the gut microbiome and cancer, which include cancer pathogenesis and progression through regulation of tumor immune surveillance [53, 54]. For example, in colorectal cancer (CRC), the gut microbiota metabolites deoxycholic acid (DCA) and lithocholic acid (LCA) may contribute to CRC development through modulation of cell signaling cascades and membrane perturbation effects [55, 56]. In lung cancer, the gut microbiota promotes lung cancer development and progression through regulation of metabolic pathways, suppression of immune cell function, and production of pro-inflammatory factors [57].
The gut microbiota plays a key role in diseases such as cardiovascular disease, atherosclerosis, hypertension, diabetes mellitus, and inflammatory bowel disease (IBD) [58,59,60,61,62]. It influences the course of disease by participating in and regulating numerous metabolic processes in the host, including energy homeostasis, glucose metabolism, and lipid metabolism [63]. For example, in IBD, imbalances in microbial homeostasis contribute to the development of IBD by increasing the risk of host immune responses [64]. In the case of hypertension, it triggers an imbalance in the intestinal flora as well as intestinal barrier dysfunction, as evidenced by an increase in harmful bacteria, hydrogen sulfide, and lipopolysaccharides (LPS), a decrease in beneficial bacteria and SCFAs, a decrease in intestinal tight junction proteins, and an increase in intestinal permeability [65]. The gut microbiota has been the subject of targeted therapies against dyslipidemia-related diseases [66]. This is sufficient to confirm the importance of the gut microbiota in therapy.
In conclusion, the gut microbiota is not only associated with leukemia, but also causally involved in both neoplastic and non-neoplastic diseases, either directly or indirectly influencing the course of the disease. Insights gained show that the homeostasis of the gut microbiota and the metabolites of the flora should not be underestimated in the treatment of leukemia.
Gut Microbiota and Metabolites
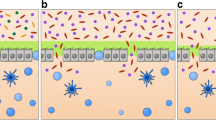
Bacterial metabolites of the intestinal microbiota including SCFAs, acetate (C2), propionate (C3), and butyrate (C4)—particularly SCFAs and butyrate—are produced by thick-walled intestinal bacteria [67]. Butyrate demonstrates a localized protective effect on the intestinal barrier, exerts a protective effect on the immune system through macrophage activation and regulatory T-cell development, and also plays a key role in controlling inflammation [67, 68]. In addition, butyrate affects gene expression through the inhibition of histone deacetylase (HDAC) and activation of histone demethylase LSD1 [69]. Research has shown that AML contributes to the development of intestinal dysbiosis. It has been suggested that AML leads to impaired intestinal integrity, reduced barrier function, butyrate deficiency, and elevated blood LPS levels, which in turn accelerates the progression of AML. In contrast, increased production of SCFAs (especially butyrate) and activation of G protein-coupled receptors (GPRs) such as GPR43 and GPR109A, which regulate peripheral blood LPS concentrations by affecting the intestinal barrier, may slow the progression of AML [70]. Lactic acidosis has been reported in patients during the development of leukemia [71,72,73]. The Warburg effect has been associated with many malignancies, of which hematologic malignancies, such as leukemia, are the most common [74, 75]. Activation of G protein-coupled receptor 81 (GPR81) by lactate or shuttling to the membrane via the monocarboxylate transporter protein (MCT) controls immune evasion and chemotherapeutic drug resistance [76, 77] (Fig. 1). These findings confirm the association of gut microbiota metabolites with immune regulation in patients with leukemia. However, the modes and mechanisms of regulation are still unclear.
Butyrate in short-chain fatty acids plays multiple physiological roles by regulating gene expression in intestinal cells through inhibition of HDAC and other pathways. Butyrate induces regulatory T cells (Treg) expressing the transcription factor Foxp3 via DC, and propionate activates with GPRs to inhibit HDAC and indirectly produce IL-10. Cytokine activation of the IL-25 receptor upregulates the expression of Th-2-related cytokines, which indirectly promotes the differentiation and expansion of Th-2 cells. The lactate–GPR81–Wnt/ß–catenin axis regulates jejunal stem cell function and promotes proliferation of intestinal epithelial cells and RX3CP1-mediated phagocytosis for tissue repair and regeneration. CSFAs short-chain fatty acids; GPRs G protein-coupled receptors; GPR109a G protein-coupled receptor 109a; GPR81 G protein-coupled receptor 81; GPR31 G protein-coupled receptor 31; Wnt3 a member of the Wnt family of proteins involved in the classical Wnt/ß-catenin signaling pathway; HDAC histone deacetylases; IL-10 interleukin 10; IL-25 interleukin 25; Th-2 type 2 helper T cells; DC dendritic cells; Foxp3+Treg regulatory T cells (Treg) expressing the transcription factor Foxp3
Gut Microflora and Leukemia Therapy
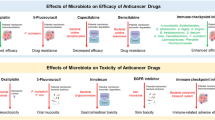
Chemotherapy, infection prevention, and drug control remain the mainstay of treatment associated with AL. Chemotherapy is a systemic cancer treatment utilizing cytotoxic drugs that significantly improves the overall survival of patients [78]. The relationship between chemotherapeutic agents and the gut microbiota is interactive during treatment. Chemotherapeutic agents may cause imbalances by altering the gut microbial ecology or causing damage to the gut epithelium. Similarly, imbalances in the gut microbiota may affect the absorption and metabolism of chemotherapeutic agents, resulting in increased toxicity and decreased efficacy [16]. Recent studies on the course of leukemia treatment with regard to the gut microbiota are summarized in Table 3.
In pharmacological microbiology, certain intestinal microbiota can alter the structure of a drug through enzymatic action, thereby altering the bioavailability, bioactivity, or toxicity of the drug, which in turn affects the individual's response to the drug [79]. Changes in the gut microbiota of patients with AL can also directly affect the body's absorption and response [16].
Methotrexate (MTX) is an important antitumor drug widely used for the treatment of leukemia by inhibiting folate metabolism in tumor cells, which can cause significant damage to the intestinal tract [80, 81]. In addition, the damage caused by MTX to intestinal epithelial cells induces a series of inflammatory responses. Animal experiments have shown that MTX-treated mice display reduced intestinal microbial diversity and changes in the composition of the intestinal microbiota with Bacteroides fragilis, and there is a tendency for B. fragilis to proliferate at a reduced rate as the density of M1 macrophages increases [82].
Cyclophosphamide (CTX) has potent anticancer activity and can be effective in the treatment of AML. Viaud et al. investigated the role of gut microbiota in CTX therapy using mouse models [83], and noted that CTX causes damage to the intestinal epithelial barrier, which is manifested by discontinuity of the epithelial barrier, shortening of villi, and mesenchymal edema [83]. They also found that treatment with CTX had a significant impact on the intestinal epithelial barrier. In addition, in mice treated with CTX, commensal bacteria from the gut microbiota (e.g., Lactobacillus johnsonii, Lactobacillus rhamnosus, and E. faecalis) were detected in lymphoid organs such as mesenteric lymph nodes and spleen due to the impairment of the intestinal barrier. This suggests that a significant translocation of the gut microbiota occurred [83]. It was also reported that CTX treatment induced selective translocation of the gut microbiota, resulting in reduced abundance of Lactobacillus and Enterococcus in the small intestine of mice [50].
Bortezomib has been shown to be effective in relapsed T-cell acute lymphoblastic leukemia (T-ALL) [84], while Fusobacterium citriodora is associated with multiple myeloma (MM) relapse and induces bortezomib resistance [85]. Of interest, other novel drugs targeting leukemia cells, such as the FLT3 inhibitor gilteritinib, have shown promising efficacy in treating relapsed or refractory AML harboring FLT3 mutations [86]. Venetoclax, a BCL-2 inhibitor, and inotuzumab ozogamicin, an antibody–drug coupling agent, are currently missing from studies on the association with gut microbes.
Both MTX and CTX cause damage to the intestinal mucosa, resulting in increased intestinal permeability, which in turn leads to dysbiosis of the gut microbiota, resulting in altered blood flow and exacerbating the progression of leukemia. However, there is a lack of research on novel drugs targeting leukemia cells and the gut microbiota. Therefore, the effects of chemotherapeutic agents on the ecological stability of the gut still require extensive research, and the potential effects of different chemotherapeutic agents need to be taken into account in clinical treatment.
Association Between Gut Microbiota and Leukemia Complications
The gut microbiota and the host exist in dynamic equilibrium, and when this equilibrium is disrupted, it can lead to the onset or progression of host disease and complications. Studies have shown that complications after chemotherapy are associated with alterations in the gut microbiota and affect the prognosis of patients with leukemia. Major complications from chemotherapy include gastrointestinal dysfunction, neutropenia, infection, and organ toxicity. Typically, the earliest and only infectious complication in patients with AL is neutropenic fever (febrile neutropenia, FN). In some studies, Gram-negative bacteria are the main cause of infections in hematologic patients with NF tumors, and multidrug resistance has emerged among these pathogens [87]. Carbapenem resistance has been found in more than 50% of the major pathogens isolated from neutropenic patients (i.e., E. coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae), and up to 50% of Enterobacteriaceae isolates in some European countries [88, 89].
It was found that the gut microbiota of patients with NF is highly diverse, and patients with AML presenting with NF for the first time postoperatively had a significantly reduced gut flora, which remained unchanged despite bone marrow recovery or remission [90]. Among the common complications in patients with hematologic malignancies receiving chemotherapy, NF is associated with high morbidity and mortality [35]. It is particularly important to prevent the possible development of NF in patients with leukemia during chemotherapy in which antibiotics are applied. Antibiotics can cause damage to the intestinal mucosa, leading to dysbiosis of the intestinal microbiota and impairment of the intestinal barrier. This is similar to recurrent Clostridium difficile infection, where the gut microbiota response to antibiotics depends on the baseline microbiota [91]. Disturbances in gut microbiota-to-host interactions during periods of antibiotic use can lead to infections and immune dysregulation [92]. There is also significant variability in the gut microbiota of patients with leukemia in chemotherapy remission without prophylactic antimicrobial agents or NFs [93], such as fecal butyric acid-producing cocci, fecal mimics, and butyric acid-producing bacteria.
However, dysbiosis of the gastrointestinal microbiota may result during intensive chemotherapy through direct damage to the gut and indirect effects on the immune system, and therapeutic or prophylactic antibiotics used during chemotherapy may also affect the gut microbiota. In addition, instability of the gut ecology during repeated treatment may lead to dysbiosis of the gut microbiota [94]. Therefore, the use of antibiotics during chemotherapy is essential. A previous study showed that ciprofloxacin prevented NF, was well tolerated, and had no serious side effects [95]. However, the durability of the protective effect of antibiotics remains to be investigated.
Association Between Gut Microbiota and Infection Risk
Disruption of the gut microbiota results in the incursion of pathogenic bacteria such as E. coli, Akkermansia muciniphila, and B. fragilis, and induces inflammation. A previous study revealed a significant difference in the gastrointestinal microbiota between children with ALL and healthy controls, as analyzed by 16S rRNA gene sequencing, and this difference was observed both before and after chemotherapy, which significantly reduced the diversity of the gut microbiota and some probiotic strains [33, 96]. This can increase the risk of bloodstream infections and even some complications in patients. It has been shown that altered gut microbiota induces pneumonia related to pediatric ALL [97]. The gut microbiota of children with ALL receiving chemotherapy is dominated by Enterococcaceae and Streptococcaceae, which predicts the development of infections during the subsequent treatment phase [35]. For example, in mouse models of ALL, severe damage to gut-associated lymphoid tissues led to lymphocytopenia and ultimately to bloodstream infections [98]. In contrast, bloodstream infections are also caused by an imbalance in the intestinal flora, resulting in damage to the intestinal mucosal and epithelial barriers. Part of the intestinal flora then enters the bloodstream or local lymph nodes, leading to an inflammatory immune response through metabolic disturbance, immune cell activation, and alterations in key intracellular signaling pathways, which may play a role in tumor development [90].
Curcumin treatment in leukemia improves intestinal integrity and modulates the gut microbiota response to cytarabine, and inhibits inflammation and improves chemotherapy resistance in patients with AL [99]. In a 16S rRNA-based analysis of patient feces in a study of 97 patients with AML, the alpha diversity of the gut microbiota at baseline predicted the risk of infection during chemotherapy [44]. Immunocompromised patients with leukemia are at increased risk for infection, especially related to multidrug resistance. Intestinal decontamination (ID) and selective intestinal decontamination (SID) aim to remove potentially pathogenic microorganisms from the gastrointestinal tract, especially Gram-negative aerobic bacilli, and thereby prevent infectious complications [100]. Early studies have shown that SID significantly reduces the colonization of Gram-negative aerobic bacteria and Candida in the gut, which in turn reduces the risk of infection [101]. In the case of ID of multidrug-resistant Klebsiella pneumoniae in immunocompromised patients with leukemia after recurrent infections, it was shown that follow-up monitoring of stool cultures for MDR and carbapenem-resistant Klebsiella pneumoniae at 36 and 58 days after bowel decontamination was negative, and the patient's hematopoietic stem cell transplantation (HSCT) was successful without recurrence of leukemia [102]. However, studies of the effects of SID on the gut flora have shown an increase in the level of aminoglycoside resistance genes in commensal microorganisms [103, 104], and widespread use is bound to increase antibiotic resistance and alter the structure of pathogenic microorganisms [105]. Nevertheless, most studies conducted to date have found that SID is effective in reducing Gram-negative infections. Therefore, by analyzing the characteristics of the patient's flora to determine whether the patient is exposed to further infections and taking appropriate prophylactic measures, the rational use of SID may prevent risk of infection and reduce patient morbidity and mortality.
Gut Microbiota and Hematopoietic Stem Cell Transplantation
It has been shown that recurrent C. difficile infection in patients with leukemia undergoing HSCT leads to dysbiosis of the intestinal microflora, which can be corrected by fecal microbiota transplantation (FMT), a safe and effective method for treating C. difficile infection after HSCT [106]. HSCT is an effective treatment for patients with relapsed or refractory ALL. Previous studies have shown that changes in intestinal flora after HSCT are strongly associated with major complications such as graft-versus-host disease (GvHD), infection, and relapse. Recent studies have found that certain gut bacteria are associated with the outcome of allogeneic HSCT (allo-HSCT) transplantation, and disruption of the normal structure of the gut microbiota is associated with the risk of GvHD [107, 108].
GvHD is a pro-inflammatory syndrome induced by donor T cells and includes both acute (aGvHD) and chronic (cGvHD) types [109]. Studies have shown that severe GvHD is associated with increased abundance of Enterobacteriaceae, while C. difficile is associated with an anti-inflammatory response [110]. In allo-HSCT, GvHD causes mucosal damage in the gastrointestinal tract, which in turn leads to intestinal microecological disorders and a decrease in flora. According to the literature, the progression of GvHD promotes the damage to intestinal stem cells, which ultimately leads to intestinal mucosal damage [111]. Mucosal damage and intestinal dysbiosis increase the incidence of GvHD and associated mortality, which is significantly corrected with the restoration of the intestinal flora [112]. Sofi et al. demonstrated that stabilization of the intestinal microflora could reduce the incidence of GvHD by stabilizing the intestinal ecosystem with B. fragilis [113].
Taur et al. investigated the effect of gut diversity on post-transplant mortality, suggesting that the gut flora are a key factor in the success or failure of allo-HSCT [114]. In a cohort study of 80 patients with leukemia undergoing HSCT, lower gut flora diversity was associated with higher mortality, suggesting that microbial diversity is an important predictor of mortality [114]. In addition, alpha diversity of the intestinal flora was associated with mobility characteristics in pediatric patients with ALL treated with HSCT and undergoing an exercise training program [115]. Furthermore, the relationship between gene therapy and clinical outcomes is the strongest evidence for adult allo-HSCT [38]. HSCT-related events and patient immunity are associated with specific alterations in the gut microbiome [116]. For example, the genus Bifidobacterium, a beneficial bacterium that produces butyric acid, was significantly reduced in patients prior to receiving allo-HSCT, and these bacteria have been reported to attenuate the worsening of intestinal inflammation [117]. In a small-sample prospective study evaluating changes in the gut microbiome of patients with MM in the first year after autologous HSCT, a reduction in gut microbial alpha diversity at the time of cell implantation was associated with autologous HSCT tolerance [118]. In addition, fecal microbiota diversity in patients with lymphoma, MM, and amyloidosis treated with autologous HSCT was lower early before transplantation and further decreased during transplantation. For patients with MM and a relatively high level of diversity, the risk of disease progression or death during neutrophil transplantation is lower [116, 119]. A study including 13 patients with AL treated with HSCT showed that the gut microbiota plays an important role in the regulation of muscle metabolism [120]. Therefore, the diversity or composition of the gut microbiota or specific species may serve as potential biomarkers for predicting and preventing complications after allogeneic or autologous HSCT and prolonging the duration of transplantation.
Gut Microbiota and Immunotherapy
In recent years, the efficacy of chemotherapy and HSCT for the treatment of leukemia has improved significantly, but problems such as primary or secondary drug resistance, relapse after remission, and serious complications still exist. Novel cancer immunotherapies with high specificity and low toxicity have shown great potential in the treatment of refractory or relapsed leukemia. Currently, the major immunotherapies for the treatment of leukemia include chimeric antigen receptor (CAR) T-cell therapy, antibody–drug coupling (ADC), bispecific T-cell inducer (BiTE) therapy, immune checkpoint inhibitors (ICIs), natural killer (NK) cell therapy, and dendritic cell (DC) therapy. There is growing evidence that the microbiota may alter the response to cancer immunotherapy. A study of immunotherapy utilizing CAR T-cell therapy for relapsed/refractory MM (n = 43), ALL (n = 23), and non-Hodgkin lymphoma (NHL; n = 12) demonstrated a high success rate for the gut microbiota [121]. It was found that 20(S)-GRh20, which affects the immune system and gut microbiota, altered the composition of the gut microbiota by increasing the levels of tight junction proteins, antimicrobial peptides, and immunoglobulin A (IgA), promoted gut homeostasis, and ameliorated LPS-induced intestinal inflammatory responses in T-ALL mice [122]. ICIs prevent molecules such as CTLA4, PD-1/PD-L1, LAG-3, and TIM-3 from binding to their ligand receptors, activate the suppressed immune system, and promote the clearance of malignant cells [123]. The gut microbiota was shown in one study to have a significant impact on the gut microbiota. The composition of the gut microbiota (different Anabaena spp.) was found to affect the antitumor effects of CTLA-4 resistance in tumor-bearing mice in vivo, and the antitumor effect of CTLA-4 blockade was dependent on different Anabaena spp. [123]. It was shown in a mouse model of hepatocellular carcinoma that vancomycin-induced alterations in the gut microbiota may exert anticancer effects by increasing the number of hepatic NK T cells and decreasing the level of secondary bile acid metabolism [124]. Therefore, gut microbiota and flora interactions should be further explored in vitro and in vivo to improve the efficacy of immunotherapy.
Gut Microbiota and Fecal Microbiota Transplantation
FMT is a therapeutic approach in which a fecal suspension containing healthy donor microbiota is injected into the gastrointestinal tract of a recipient to improve the composition and function of the host's intestinal flora, which can be tailored to the individual patient [125,126,127]. Previous studies have confirmed the successful use of FMT to treat steroid-refractory aGvHD [128, 129]. FMT has been reported to be more effective than various probiotics in repairing the intestinal flora in animal models using antibiotics [130]. In a clinical trial of four patients with AML undergoing allo-HSCT who developed refractory diarrhea due to intestinal infections or intestinal GVHD, FMT proved to be a successful and safe therapy [131]. In a study using mice as a model, antibiotic-induced intestinal bacterial imbalance promoted AML, whereas butyrate obtained from fecal transplants slowed the progression of AML and improved intestinal mucosal barrier function [70]. In a study of autologous fecal transplant therapy (aFMT) in 25 patients with AML treated with intensive chemotherapy and antibiotics, aFMT appeared to be safe and effective in restoring the gut microbiota [132]. In addition, FMT after HSCT promotes the reconstitution of gut flora structure and bacterial architecture with better efficacy [133, 134]. The results of this study are summarized in Table 4. As research progresses, studies have increasingly shown that dysbiosis of the gut flora plays an important role in several neurological disorders, including Parkinson's disease (PD) [21]. Furthermore, in a prospective study, 16 patients with PD were treated with FMT, and the balance of gut bacteria in these patients normalized [135]. Therefore, FMT can quickly and effectively restore the disrupted intestinal flora, and its efficacy has been confirmed in a large number of clinical studies.
Gut Microbiota and Probiotics/Prebiotics
Probiotics are active microorganisms that stabilize the intestinal ecosystem, promote nutrient absorption, protect the intestinal mucosal barrier, and modulate immunity. In a randomized controlled trial involving 60 children with AL receiving chemotherapy, patients in the probiotic group reported significantly fewer gastrointestinal side effects [136]. A systematic evaluation of probiotics has shown a significant reduction in gastrointestinal side effects as well, with a reduction in the frequency and severity of diarrhea in patients with cancer [137]. However, some studies have shown that probiotics are not effective for cancer complications such as diarrhea [138]. Therefore, treatment with probiotics cannot be generalized, and the mechanism of action may be related to the patient's tolerance level.
Prebiotics are found in certain foods, such as soybean extract, and have an indirect effect by altering the composition of the gut flora. For example, a soy–whey mixture significantly increased the diversity of the gut microbiota and was effective in improving muscle condition in AL survivors [120]. A study in mice with allogeneic bone marrow transplantation showed that the addition of a soy–whey protein mixture accelerated the recovery of hematopoietic function and the reconstitution of immune function [139]. In addition, polysaccharides and ginsenosides extracted from American ginseng were found to be protective against CTX therapy [140]. Enteral nutrition can rapidly restore the balance of gut microbiota after HSCT [80]. Therefore, moderate addition of prebiotics is another therapeutic approach to improve the structure of the intestinal flora.
Discussion
In the coming years, the study of the relationship between the gut microbiota and leukemia is expected to achieve more in-depth and comprehensive results. With advances in technology, such as more advanced sequencing techniques and more precise analytical methods, we will be able to more clearly reveal the complex causal relationship between the gut microbiota and the development of leukemia.
On the therapeutic side, gut microbiota-based therapies such as FMT, probiotics, and prebiotics, which we summarized through ClinicalTrials.gov, are still relatively sparse in ongoing or completed studies in recent years (Table 4), but are expected to be further optimized and standardized. More large-scale, multicenter clinical trials will provide stronger evidence for the efficacy and safety of these therapies, leading to more personalized treatment options for patients with leukemia.
With regard to the role of the gut microbiota in leukemia complications, future studies will focus on clarifying the specific mechanisms and regulatory pathways, leading to the development of more targeted preventive and therapeutic strategies to reduce the incidence and severity of complications.
In the field of immunotherapy, the importance of the gut microbiota is already being demonstrated [141]. By gaining a deeper understanding of how the gut microbiota affects the immune system's response to leukemia, it is expected that combination therapies will be developed to improve the efficacy of immunotherapy. In addition, with a deeper understanding of the interaction between the gut microbiota and leukemia, interdisciplinary collaborations will become closer, including experts in microbiology, immunology, oncology, and other fields working together to promote the translation of basic research into clinical applications. This will bring new breakthroughs in the treatment of leukemia and improve the survival rate and quality of life for patients.
Conclusion
In conclusion, in this study, the intestinal flora were explored in depth and found to be strongly associated with tumorigenesis, tumor growth, HSCT, and chemotherapy efficacy. Thus, modulation of the gut flora supplemented with appropriate interventions is expected to revolutionize the treatment and management of leukemia in the future.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Pelcovits A, Niroula R. Acute myeloid leukemia: a review. R I Med J. 2013;2020(103):38–40.
Juliusson G, Hough R. Leukemia. Prog Tumor Res. 2016;43:87–100.
Bispo JAB, Pinheiro PS, Kobetz EK. Epidemiology and etiology of leukemia and lymphoma. Cold Spring Harb Perspect Med. 2020;10: a034819.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020 GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. https://doi.org/10.3322/caac.21660.
Steliarova-Foucher E, Colombet M, Ries LAG, Moreno F, Dolya A, Bray F, et al. International incidence of childhood cancer, 2001–10: a population-based registry study. Lancet Oncol. 2017;18:719–31.
Chiorazzi N, Chen S-S, Rai KR. Chronic lymphocytic leukemia. Cold Spring Harb Perspect Med. 2021;11: a035220.
Kikushige Y. Pathogenesis of chronic lymphocytic leukemia and the development of novel therapeutic strategies. J Clin Exp Hematop JCEH. 2020;60:146–58.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am J Hematol. 2022;97:1236–56.
Shah NP, Bhatia R, Altman JK, Amaya M, Begna KH, Berman E, et al. Chronic myeloid leukemia, version 2. 2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. 2024;22:43–69.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28.
Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–5.
Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev MMBR. 2017;81:e00036-e117.
Arrieta M-C, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427.
Cheng J, Ringel-Kulka T, Heikamp-de Jong I, Ringel Y, Carroll I, de Vos WM, et al. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016;10:1002–14.
Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta T-A, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3:36.
Zhou Y, Zhou C, Zhang A. Gut microbiota in acute leukemia: current evidence and future directions. Front Microbiol. 2022;13:1045497.
Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904.
Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31:69–75.
Zapata HJ, Quagliarello VJ. The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc. 2015;63:776–81.
Maeda Y, Takeda K. Host-microbiota interactions in rheumatoid arthritis. Exp Mol Med. 2019;51:1–6.
Kaur G, Behl T, Bungau S, Kumar A, Uddin MS, Mehta V, et al. Dysregulation of the gut-brain axis, dysbiosis and influence of numerous factors on gut microbiota associated Parkinson’s disease. Curr Neuropharmacol. 2021;19:233–47.
Yu L-X, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527–39.
Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52:241–55.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65.
Mandal RS, Saha S, Das S. Metagenomic surveys of gut microbiota. Genom Proteom Bioinform. 2015;13:148–58.
Riley DR, Sieber KB, Robinson KM, White JR, Ganesan A, Nourbakhsh S, et al. Bacteria-human somatic cell lateral gene transfer is enriched in cancer samples. PLoS Comput Biol. 2013;9: e1003107.
Allegra A, Innao V, Allegra AG, Ettari R, Pugliese M, Pulvirenti N, et al. Role of the microbiota in hematologic malignancies. Neth J Med. 2019;77:67–80.
Guevara-Ramírez P, Cadena-Ullauri S, Paz-Cruz E, Tamayo-Trujillo R, Ruiz-Pozo VA, Zambrano AK. Role of the gut microbiota in hematologic cancer. Front Microbiol. 2023;14:1185787.
de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71:1020–32.
Nakkarach A, Foo HL, Song AA-L, Mutalib NEA, Nitisinprasert S, Withayagiat U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact. 2021;20:36.
Cheng WY, Wu C-Y, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69:1867–76.
Thomas R, Wong WSW, Saadon R, Vilboux T, Deeken J, Niederhuber J, et al. Gut microbial composition difference between pediatric ALL survivors and siblings. Pediatr Hematol Oncol. 2020;37:475–88.
De Pietri S, Ingham AC, Frandsen TL, Rathe M, Krych L, Castro-Mejía JL, et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int J Cancer. 2020;147:1953–62.
Sly LM. Gut microbes in pediatric ALL survivorship. Pediatr Hematol Oncol. 2020;37:451–4.
Hakim H, Dallas R, Wolf J, Tang L, Schultz-Cherry S, Darling V, et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:541–8.
Vicente-Dueñas C, Janssen S, Oldenburg M, Auer F, González-Herrero I, Casado-García A, et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood. 2020;136:2003–17.
Marcotte EL, Richardson MR, Roesler MA, Spector LG. Cesarean delivery and risk of infant leukemia: a report from the Children’s Oncology Group. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2018;27:473–8.
Masetti R, Muratore E, Leardini D, Zama D, Turroni S, Brigidi P, et al. Gut microbiome in pediatric acute leukemia: from predisposition to cure. Blood Adv. 2021;5:4619–29.
Li H, Sun B, Ning X, Jiang S, Sun L. A comparative analysis of Edwardsiella tarda-induced transcriptome profiles in RAW264.7 cells reveals new insights into the strategy of bacterial immune evasion. Int J Mol Sci. 2019;20:5724.
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15.
Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167:1897.
Faitová T, Svanberg R, Da Cunha-Bang C, Ilett EE, Jørgensen M, Noguera-Julian M, et al. The gut microbiome in patients with chronic lymphocytic leukemia. Haematologica. 2022;107:2238–43.
Kawari M, Akhtar M, Sager M, Basbous Z, Baydoun I, Kabanja J, et al. Alterations of gut microbiome in untreated chronic lymphocytic leukemia (CLL) future therapeutic potentials. Blood. 2019;134:5455. https://doi.org/10.1182/blood-2019-121643.
Galloway-Peña JR, Shi Y, Peterson CB, Sahasrabhojane P, Gopalakrishnan V, Brumlow CE, et al. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin Infect Dis. 2020;71:63–71.
Galloway-Peña JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, et al. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122:2186–96.
Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Halaweish HF, Kaiser T, et al. Lasting shift in the gut microbiota in patients with acute myeloid leukemia. Blood Adv. 2022;6:3451–7.
Pötgens SA, Lecop S, Havelange V, Li F, Neyrinck AM, Neveux N, et al. Gut microbiota alterations induced by intensive chemotherapy in acute myeloid leukaemia patients are associated with gut barrier dysfunction and body weight loss. Clin Nutr. 2023;42:2214–28.
Yu D, Yu X, Ye A, Xu C, Li X, Geng W, et al. Profiling of gut microbial dysbiosis in adults with myeloid leukemia. FEBS Open Bio. 2021;11:2050–9.
van den Bogert B, Erkus O, Boekhorst J, de Goffau M, Smid EJ, Zoetendal EG, et al. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol Ecol. 2013;85:376–88.
Ma T, Chen Y, Li L-J, Zhang L-S. Opportunities and challenges for gut microbiota in acute leukemia. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.692951.
Almuhayawi MS, Mohamed MSM, Abdel-Mawgoud M, Selim S, Al Jaouni SK, AbdElgawad H. Bioactive potential of several actinobacteria isolated from microbiologically barely explored desert habitat. Saudi Arab Biol. 2021;10:235.
Li Z, Ke X, Zuo D, Wang Z, Fang F, Li B. New insights into the relationship between gut microbiota and radiotherapy for cancer. Nutrients. 2022;15:48.
Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med. 2023;21:66.
Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res CR. 2021;40:221.
Qu R, Zhang Y, Ma Y, Zhou X, Sun L, Jiang C, et al. Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv Sci Weinh Baden-Wurtt Ger. 2023;10: e2205563.
Wang Z, Dan W, Zhang N, Fang J, Yang Y. Colorectal cancer and gut microbiota studies in China. Gut Microbes. 2023;15:2236364.
Zhao Y, Liu Y, Li S, Peng Z, Liu X, Chen J, et al. Role of lung and gut microbiota on lung cancer pathogenesis. J Cancer Res Clin Oncol. 2021;147:2177–86.
Velasquez MT, Centron P, Barrows I, Dwivedi R, Raj DS. Gut microbiota and cardiovascular uremic toxicities. Toxins. 2018;10:287.
Luqman A, Hassan A, Ullah M, Naseem S, Ullah M, Zhang L, et al. Role of the intestinal microbiome and its therapeutic intervention in cardiovascular disorder. Front Immunol. 2024;15:1321395.
Hemmati M, Kashanipoor S, Mazaheri P, Alibabaei F, Babaeizad A, Asli S, et al. Importance of gut microbiota metabolites in the development of cardiovascular diseases (CVD). Life Sci. 2023;329: 121947.
Zhang L, Chu J, Hao W, Zhang J, Li H, Yang C, et al. Gut microbiota and type 2 diabetes mellitus: association, mechanism, and translational applications. Mediat Inflamm. 2021;2021:5110276.
Mantziaris V, Kolios G. Gut microbiota, atherosclerosis, and therapeutic targets. Crit Pathw Cardiol. 2019;18:139–42.
Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64.
Chen L, Wang J. Gut microbiota and inflammatory bowel disease. WIREs Mech Dis. 2022;14: e1540.
Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z, et al. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens N Y N. 1993;2023(45):2195135.
Schoeler M, Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev Endocr Metab Disord. 2019;20:461–72.
Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66:1654–61.
Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23:1105.
Zhang L, Liu C, Jiang Q, Yin Y. Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol Metab. 2021;32:159–69.
Wang R, Yang X, Liu J, Zhong F, Zhang C, Chen Y, et al. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun. 2022;13:2522.
Ghrewati M, Manji F, Modi V, Chandran C, Maroules M. Severe metabolic acidemia in a patient with aleukemic leukemia. Case Rep Nephrol. 2018;2018:1019034.
Gökçe M, Unal S, Gülşen H, Başaran O, Cetin M, Gümrük F, et al. A rare metabolic complication of acute lymphoblastic leukemia in childhood: lactic acidosis. Turk J Pediatr. 2012;54:61–3.
Sayyed AH, Aleem A, Al-Katari MS, Algahtani F, Aljerian K, Aleem TA, et al. Acute lymphoblastic leukemia presenting with liver infiltration and severe lactic acidosis. Am J Case Rep. 2018;19:453–7.
Padda J, Khalid K, Kakani V, Cooper AC, Jean-Charles G. Metabolic acidosis in leukemia. Cureus. 2021;13: e17732.
Eslami M, Sadrifar S, Karbalaei M, Keikha M, Kobyliak NM, Yousefi B. Importance of the microbiota inhibitory mechanism on the Warburg effect in colorectal cancer cells. J Gastrointest Cancer. 2020;51:738–47.
Lundø K, Trauelsen M, Pedersen SF, Schwartz TW. Why Warburg works: lactate controls immune evasion through GPR81. Cell Metab. 2020;31:666–8.
Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206: 107451.
Noonan KL, Ho C, Laskin J, Murray N. The influence of the evolution of first-line chemotherapy on steadily improving survival in advanced non-small-cell lung cancer clinical trials. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2015;10:1523–31.
Lucafò M, Franzin M, Lagatolla C, Franca R, Bramuzzo M, Stocco G, et al. Emerging insights on the interaction between anticancer and immunosuppressant drugs and intestinal microbiota in pediatric patients. Clin Transl Sci. 2020;13:238–59.
Ben-Lulu S, Pollak Y, Mogilner J, Bejar J, Coran GA, Sukhotnik I. Dietary transforming growth factor-beta 2 (TGF-β2) supplementation reduces methotrexate-induced intestinal mucosal injury in a rat. PloS One. 2012;7:e45221.
Kolli VK, Abraham P, Isaac B, Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig Dis Sci. 2013;58:959–69.
Zhou B, Xia X, Wang P, Chen S, Yu C, Huang R, et al. Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBioMedicine. 2018;33:122–33.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6.
Teachey DT, Devidas M, Wood BL, Chen Z, Hayashi RJ, Hermiston ML, et al. Children’s Oncology Group trial AALL1231: a phase III clinical trial testing bortezomib in newly diagnosed T-cell acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2022;40:2106–18.
Zhu Y, Jian X, Chen S, An G, Jiang D, Yang Q, et al. Targeting gut microbial nitrogen recycling and cellular uptake of ammonium to improve bortezomib resistance in multiple myeloma. Cell Metab. 2024;36:159-175.e8.
Molica M, Perrone S, Rossi M. Gilteritinib: the story of a proceeding success into hard-to-treat FLT3-mutated AML patients. J Clin Med. 2023;12:3647.
Mikulska M, Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, et al. Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J Infect. 2014;68:321–31.
Trecarichi EM, Pagano L, Martino B, Candoni A, Di Blasi R, Nadali G, et al. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol. 2016;91:1076–81.
Escrihuela-Vidal F, Laporte J, Albasanz-Puig A, Gudiol C. Update on the management of febrile neutropenia in hematologic patients. Rev Esp Quimioter. 2019;32:55–8.
Rattanathammethee T, Tuitemwong P, Thiennimitr P, Sarichai P, Na Pombejra S, Piriyakhuntorn P, et al. Gut microbiota profiles of treatment-naïve adult acute myeloid leukemia patients with neutropenic fever during intensive chemotherapy. PLoS ONE. 2020;15: e0236460.
Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, et al. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome. 2021;9:211.
Gyssens IC, Kern WV, Livermore DM. The role of antibiotic stewardship in limiting antibacterial resistance among hematology patients. Haematologica. 2013;98:1821–5.
Shen Z, Gu X, Cao H, Mao W, Yang L, He M, et al. Characterization of microbiota in acute leukemia patients following successful remission induction chemotherapy without antimicrobial prophylaxis. Int Microbiol Off J Span Soc Microbiol. 2021;24:263–73.
Rashidi A, Kaiser T, Shields-Cutler R, Graiziger C, Holtan SG, Rehman TU, et al. Dysbiosis patterns during re-induction/salvage versus induction chemotherapy for acute leukemia. Sci Rep. 2019;9:6083.
Laoprasopwattana K, Khwanna T, Suwankeeree P, Sujjanunt T, Tunyapanit W, Chelae S. Ciprofloxacin reduces occurrence of fever in children with acute leukemia who develop neutropenia during chemotherapy. Pediatr Infect Dis J. 2013;32:e94-98.
Chen S-M, Liu S-X, Chen F, Wang C-Y, Mai H-R, Yuan X-L, et al. Changes of intestinal flora in children with acute lymphoblastic leukemia before and after chemotherapy. Zhongguo Dang Dai Er Ke Za Zhi Chin J Contemp Pediatr. 2022;24:550–60.
Liu X, Zou Y, Zhang Y, Liu L, Duan Y, Zhang A, et al. Characteristics in gut microbiome is associated with chemotherapy-induced pneumonia in pediatric acute lymphoblastic leukemia. BMC Cancer. 2021;21:1190.
Song Y, Gyarmati P. Bacterial translocation in acute lymphocytic leukemia. PLoS ONE. 2019;14: e0214526.
Liu J, Luo W, Chen Q, Chen X, Zhou G, Sun H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemother Pharmacol. 2022;89:243–53.
Resino E, San-Juan R, Aguado JM. Selective intestinal decontamination for the prevention of early bacterial infections after liver transplantation. World J Gastroenterol. 2016;22:5950–7.
Wiesner RH, Hermans PE, Rakela J, Washington JA, Perkins JD, DiCecco S, et al. Selective bowel decontamination to decrease gram-negative aerobic bacterial and Candida colonization and prevent infection after orthotopic liver transplantation. Transplantation. 1988;45:570–4.
Kronman MP, Zerr DM, Qin X, Englund J, Cornell C, Sanders JE, et al. Intestinal decontamination of multidrug-resistant Klebsiella pneumoniae after recurrent infections in an immunocompromised host. Diagn Microbiol Infect Dis. 2014;80:87–9.
Barsuk AL, Nekaeva ES, Lovtsova LV, Urakov AL. Selective intestinal decontamination as a method for preventing infectious complications (Review). Mod Technol Med. 2020;12:86–95.
Buelow E, Gonzalez TB, Versluis D, Oostdijk EAN, Ogilvie LA, van Mourik MSM, et al. Effects of selective digestive decontamination (SDD) on the gut resistome. J Antimicrob Chemother. 2014;69:2215–23.
Laupland KB, Fisman DN. Selective digestive tract decontamination: a tough pill to swallow. Can J Infect Dis Med Microbiol. 2009;20:9–11.
Webb BJ, Brunner A, Ford CD, Gazdik MA, Petersen FB, Hoda D. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem cell transplant recipients. Transpl Infect Dis Off J Transplant Soc. 2016;18:628–33.
Shono Y, van den Brink MRM. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat Rev Cancer. 2018;18:283–95.
Fredricks DN. The gut microbiota and graft-versus-host disease. J Clin Invest. 2019;129:1808–17.
Zeiser R, Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. 2017;377:2167–79.
Hong T, Wang R, Wang X, Yang S, Wang W, Gao Q, et al. Interplay between the intestinal microbiota and acute graft-versus-host disease: experimental evidence and clinical significance. Front Immunol. 2021;12: 644982.
Li J, Zhang X, Chen Y, Zheng Q, Zhao M, Jiang H. A promising insight: the potential influence and therapeutic value of the gut microbiota in GI GVHD. Oxid Med Cell Longev. 2022;2022:2124627.
Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Kazadi D, Halaweish H, et al. Potential of fecal microbiota transplantation to prevent acute graft-versus-host disease: analysis from a phase 2 trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2023;29:4920–9.
Sofi MH, Wu Y, Ticer T, Schutt S, Bastian D, Choi H-J, et al. A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight. 2021;6:e136841.
Taur Y, Jenq RR, Perales M-A, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174–82.
Ugrayová S, Švec P, Hric I, Šardzíková S, Kubáňová L, Penesová A, et al. Gut microbiome suffers from hematopoietic stem cell transplantation in childhood and its characteristics are positively associated with intra-hospital physical exercise. Biology. 2022;11:785.
Zhang L, Xiang Y, Li Y, Zhang J. Gut microbiome in multiple myeloma: mechanisms of progression and clinical applications. Front Immunol. 2022;13:1058272.
Kusakabe S, Fukushima K, Maeda T, Motooka D, Nakamura S, Fujita J, et al. Pre- and post-serial metagenomic analysis of gut microbiota as a prognostic factor in patients undergoing haematopoietic stem cell transplantation. Br J Haematol. 2020;188:438–49.
D’Angelo C, Sudakaran S, Asimakopoulos F, Hematti P, El-Gamal D, Safdar N, et al. Perturbation of the gut microbiome and association with outcomes following autologous stem cell transplantation in patients with multiple myeloma. Leuk Lymphoma. 2023;64:87–97.
Khan N, Lindner S, Gomes ALC, Devlin SM, Shah GL, Sung AD, et al. Fecal microbiota diversity disruption and clinical outcomes after auto-HCT: a multicenter observational study. Blood. 2021;137:1527–37.
Ren G, Zhang J, Li M, Tang Z, Yang Z, Cheng G, et al. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin Nutr Edinb Scotl. 2021;40:94–102.
Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13:5313.
Xia T, Zhang B, Li Y, Fang B, Zhu X, Xu B, et al. New insight into 20(S)-ginsenoside Rh2 against T-cell acute lymphoblastic leukemia associated with the gut microbiota and the immune system. Eur J Med Chem. 2020;203: 112582.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931.
Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46:171–85.
Wang J-W, Kuo C-H, Kuo F-C, Wang Y-K, Hsu W-H, Yu F-J, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc Taiwan Yi Zhi. 2019;118(Suppl 1):S23-31.
Tkach S, Dorofeyev A, Kuzenko I, Boyko N, Falalyeyeva T, Boccuto L, et al. Current status and future therapeutic options for fecal microbiota transplantation. Med Kaunas Lith. 2022;58:84.
van Lier YF, Davids M, Haverkate NJE, de Groot PF, Donker ML, Meijer E, et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med. 2020;12:eaaz8926.
Zhao Y, Li X, Zhou Y, Gao J, Jiao Y, Zhu B, et al. Safety and efficacy of fecal microbiota transplantation for grade IV steroid refractory GI-GvHD patients: interim results from FMT2017002 trial. Front Immunol. 2021;12: 678476.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174:1406-1423.e16.
Wang Q, Fu YW, Wang YQ, Ai H, Yuan FF, Wei XD, et al. Fecal microbiota transplantation for patients with refractory diarrhea after allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi Zhonghua Xueyexue Zazhi. 2019;40:853–5.
Malard F, Vekhoff A, Lapusan S, Isnard F, Dincan-Corda E, Rey J, et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat Commun. 2021;12:3084.
DeFilipp Z, Peled JU, Li S, Mahabamunuge J, Dagher Z, Slingerland AE, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv. 2018;2:745–53.
Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, Gjonbalaj M, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018;10:p9489.
Kuai X-Y, Yao X-H, Xu L-J, Zhou Y-Q, Zhang L-P, Liu Y, et al. Evaluation of fecal microbiota transplantation in Parkinson’s disease patients with constipation. Microb Cell Factories. 2021;20:98.
Reyna-Figueroa J, Barrón-Calvillo E, García-Parra C, Galindo-Delgado P, Contreras-Ochoa C, Lagunas-Martínez A, et al. Probiotic supplementation decreases chemotherapy-induced gastrointestinal side effects in patients with acute leukemia. J Pediatr Hematol Oncol. 2019;41:468–72.
Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919–29.
Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care. 2018;12:187–97.
Wu X, Hou Q, Zhao Z, Wang J, Guo Y, Lu L, et al. Effects of soy-whey protein nutritional supplementation on hematopoiesis and immune reconstitution in an allogeneic transplanted mice. Nutrients. 2022;14:3014.
Zhou R, He D, Xie J, Zhou Q, Zeng H, Li H, et al. The synergistic effects of polysaccharides and ginsenosides from American Ginseng (Panax quinquefolius L.) ameliorating cyclophosphamide-induced intestinal immune disorders and gut barrier dysfunctions based on microbiome-metabolomics analysis. Front Immunol. 2021;12:665901.
Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–8.
Rajagopala SV, Yooseph S, Harkins DM, Moncera KJ, Zabokrtsky KB, Torralba MG, et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent acute lymphoblastic leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016;17:635.
Rajagopala SV, Singh H, Yu Y, Zabokrtsky KB, Torralba MG, Moncera KJ, et al. Persistent gut microbial dysbiosis in children with acute lymphoblastic leukemia (ALL) during chemotherapy. Microb Ecol. 2020;79:1034–43.
Gao X, Miao R, Zhu Y, Lin C, Yang X, Jia R, et al. A new insight into acute lymphoblastic leukemia in children: influences of changed intestinal microfloras. BMC Pediatr. 2020;20:290.
Chua LL, Rajasuriar R, Lim YAL, Woo YL, Loke P, Ariffin H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC Cancer. 2020;20:151.
Liu X, Zou Y, Ruan M, Chang L, Chen X, Wang S, et al. Pediatric acute lymphoblastic leukemia patients exhibit distinctive alterations in the gut microbiota. Front Cell Infect Microbiol. 2020;10: 558799.
Bhuta R, DeNardo B, Wang J, Atoyan J, Zhang Y, Nelson D, et al. Durable changes in the gut microbiome in survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2021;68: e29308.
Acknowledgements
We thank Editage (www.editage.com) for their editing support on this manuscript. Thanks to FigDraw for providing a platform to create medical illustrations.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Zexin Wang and Qiang Yang wrote and structured the paper; Zexin Wang analyzed literature data and wrote the paper; Lingling Gan and Miao Liu reviewed the literature. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Qiang Yang, Zexin Wang, Miao Liu, and Lingling Gan declare that they have no competing interests. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, Q., Wang, Z., Liu, M. et al. Causal Relationship Between Gut Microbiota and Leukemia: Future Perspectives. Oncol Ther (2024). https://doi.org/10.1007/s40487-024-00300-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40487-024-00300-8