Abstract
Introduction
Limited awareness exists regarding real-world data (RWD) for palbociclib in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced/metastatic breast cancer in populations from certain countries outside of Western regions.
Methods
A systematic scoping review was conducted using PubMed and Embase to evaluate RWD for palbociclib from countries outside of Western regions that are underrepresented in clinical trials. Search criteria were aligned with our research question for relevant English-language publications, without restrictions on publication date, followed by Phase 1 (title and abstract) and Phase 2 (full-text) screening of retrieved citations as per Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Data analyses of eligible studies were done separately for abstracts and full-text publications to enhance the precision and reliability of the results.
Results
Database search yielded 1485 non-duplicate records, 46 qualified for inclusion, of which 47.8% were published as full text. The analysis of outcomes, based exclusively on full-text publications that collectively included 2048 patients treated with palbociclib, revealed the median progression-free survival (PFS) of 20.2–36.7 months, overall survival (OS) of 39.9 months (reported in one publication) and objective response rate (ORR) of 45.3–80.0% with first-line treatment. In ≥ second line, the median PFS, OS and ORR ranged from 7.0 to 24.2 months, 11 to 19.6 months, and 13.9% to 47.9%, respectively. The safety profile of palbociclib was similar to that reported in pivotal clinical studies, and no new safety concerns were identified.
Conclusions
A comprehensive volume of evidence demonstrates that palbociclib’s effectiveness and safety profile in real-world settings align with those observed in clinical trials, offering valuable insights for clinical decision-making in countries outside of Western regions underrepresented in clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is critical to understand the real-world data (RWD) for palbociclib from countries outside of Western regions, given the higher breast cancer mortality and burden in these regions associated with delayed diagnosis and restricted access to quality healthcare. |
This systematic scoping review evaluated RWD for palbociclib using PubMed and Embase databases from countries outside of Western regions that are underrepresented in clinical trials. Data analyses were conducted separately for abstracts and full-text citations to improve the accuracy of the results. |
The comprehensive analysis of 1485 non-duplicate records, narrowed down to 46 eligible full-text publications involving 2048 patients, revealed a median progression-free survival (PFS) of first-line palbociclib plus endocrine therapy (ET) treatment ranging from 20.2 to 36.7 months and an objective response rate (ORR) of 45.3–80.0%. |
In the second-line or later settings, palbociclib plus ET demonstrated a median PFS between 7.0 to 24.2 months and ORR from 13.9% to 47.9%, maintaining a safety profile in all treatment settings consistent with previous pivotal clinical studies. |
Introduction
Breast cancer is the most commonly diagnosed cancer in the world and is the fifth most common cause of cancer-related death [1]. Women account for the vast majority of breast cancer cases (approximately 99.0%) [2]. Most deaths attributed to breast cancer are caused by metastatic forms [3], and its incidence rates have been rising in Asia, Africa and South America, where historical rates were initially lower than in Western countries [4]. In 2020, nearly half (45.4%) of the 2.3 million diagnosed breast cancer cases were from Asia [5, 6].
Cyclin-dependent kinases 4 and 6 (CDK4/6) regulate cell cycle progression, and their functioning is dysregulated in several types of cancer [7, 8]. The introduction of CDK4/6 inhibitors has revolutionized the management of breast cancer [9]. In 2015, palbociclib, in combination with letrozole, was the first CDK4/6 inhibitor to be approved by the US Food and Drug Administration (FDA) for the treatment of metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer [8]. Subsequently, two other CDK4/6 inhibitors, ribociclib and abemaciclib, were also approved in the USA (both in 2017) for this indication [8].
The National Comprehensive Cancer Network (NCCN) guidelines recommend palbociclib as part of first-line therapy in combination with an aromatase inhibitor (AI) or fulvestrant for the treatment of HR-positive, HER2-negative, recurrent, unresectable or stage IV breast cancer in postmenopausal women or in premenopausal women receiving ovarian ablation or suppression [10]. In addition, palbociclib is recommended in the same patient population as second- and subsequent-line therapy in combination with fulvestrant if a CDK4/6 inhibitor has not been previously used [10]. The pan-Asian adaptation of the European Society for Medical Oncology (ESMO) guidelines recommends palbociclib for the treatment of HR-positive, HER2-negative metastatic breast cancer in the first-line setting in combination with an AI [11]. These recommendations are based, in large part, on the findings of PALOMA-2 and PALOMA-3 randomized controlled trials (RCTs) [11]. Much of what is currently known about the real-world effectiveness, safety profile and patterns of use of palbociclib is based on research conducted in Western contexts [12,13,14,15,16,17,18,19,20,21,22]. However, recent regulatory requirements related to Equity, Diversity and Inclusion (EDI) are likely to improve recruitment from currently underrepresented geographies [23].
Real-world data (RWD) are derived from actual clinical practice and can offer insights on outcomes in heterogeneous patient populations that may not have been eligible for inclusion in clinical trials and, as a result, can better inform clinical practice [24]. However, the availability of RWD for palbociclib, especially from countries outside of Western regions, remains limited. Breast cancer mortality and burden in terms of disability-adjusted life-years is higher in low- and medium-income countries [25, 26], often because of delayed diagnoses and limited access to quality treatment and care [4, 5]. Therefore, such data can be crucial in understanding regional variations in clinical practice and treatment outcomes [27].
The objective of this scoping review was to review the published real-world evidence on the effectiveness and safety of palbociclib in patients with HR-positive, HER2-negative advanced breast cancer in countries outside of Western regions that are underrepresented in clinical trials.
Methods
The present review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, Extension for Scoping Reviews (PRISMA-ScR) [28].
Literature Search
The literature search was conducted on August 11, 2023, using two prominent biomedical databases: PubMed (National Library of Medicine, Bethesda, MD, USA) and Embase (Elsevier, Amsterdam, The Netherlands). PubMed was chosen because it indexes a large number of academic journals and is widely used in the biomedical community, while Embase was chosen because it has a broader international scope and includes an extensive collection of conference presentations, which is particularly relevant for the purposes of this review.
The terms used for searching both PubMed and Embase were constructed based on three concepts:
-
Concept 1: real-world evidence;
-
Concept 2: palbociclib;
-
Concept 3: breast cancer.
Currently, there is an absence of specific Medical Subject Headings (MeSH) for RWD; hence, we adapted our search terms and keywords based on existing literature and our research objectives. Most of the related MeSH terms and keywords were specifically derived from the comprehensive publication ‘A Conceptual Search Filter to Identify Real-World Evidence’ published in Value in Health [29]. Additionally, we expanded our search to include synonyms for the term ‘real-world evidence’ by reviewing published scoping and systematic reviews on this topic [30,31,32], while alternative terms and brand names used for palbociclib were identified from the publicly available version of the AdisInsight database (Springer Nature, Berlin, Germany) [33, 34]. In addition, database-specific controlled vocabulary was used. Full search terms are provided in Supplementary Methods. Search results were limited to English-language publications, but no limits on the date of publication were applied.
The abstracts of all publications identified from the systematic search were uploaded onto the Covidence software (Veritas Health Innovation Ltd, Melbourne, Australia) for screening and data extraction.
In addition to the systematic search strategy implemented in PubMed and Embase, a manual search was conducted to identify relevant citations that may have been missed by these two databases or were published subsequently to the search date. The studies identified through this manual search were not included in the final count of studies or in the quantitative synthesis of the results. Instead, they are presented separately to provide additional context, allow for comparison or elaborate on the findings derived from data from the systematic search.
Geographic Scope
The objective of this review was to evaluate the RWD on the use of palbociclib in patients with HR-positive, HER2-negative advanced or metastatic breast cancer in countries outside of Western regions that are underrepresented in clinical trials. Therefore, countries of North America (Canada and the USA), Europe, Australia and New Zealand [35, 36] were excluded. Furthermore, among countries outside of Western regions, nations such as China, Japan and South Korea are recognized as large, developed economies with robust healthcare infrastructures. A substantial body of RWD on the use of palbociclib in these countries already exists [37,38,39,40]. As a result, China, Japan and South Korea were also excluded. Russia and Turkey are transcontinental nations bridging Europe and Asia [41]; they have advanced healthcare infrastructures and active research domains [42,43,44,45]. However, the body of RWD on palbociclib from these countries remains relatively limited. This limitation may arise from a variety of factors, including differences in drug approval timelines, healthcare policies and patient access to new therapies [6, 46]. Therefore, both Russia and Turkey were included.
Based on these considerations, the geographic scope of this review was defined to include countries of Latin America, Africa and the Middle East region, as well as Asian countries, including, Russia and Turkey. Studies from three Asian countries (China, Japan and South Korea) and from all Western countries (Australia, Canada, Europe, New Zealand and the USA) were defined as outside the scope of this review.
Study Eligibility and Screening
The publications identified from the literature search were subjected to a two-phase screening process. In phase 1, the titles and abstracts of all identified publications were reviewed against the eligibility criteria. Publications that met the eligibility criteria during phase 1 screening proceeded to phase 2 screening. In phase 2, the full text of candidate publications was reviewed. The screening was conducted by two reviewers in parallel.
Eligible publications described real-world studies conducted in adult patients of either sex who had HR-positive, HER2-negative advanced or metastatic breast cancer and were treated with palbociclib plus an AI as first-line therapy or with palbociclib plus fulvestrant following disease progression on endocrine therapy (ET, regardless of the previous chemotherapy) from relevant countries outside of Western regions. Studies that evaluated the effectiveness, safety and/or tolerability, quality of life or patient-reported outcomes (PRO) with palbociclib were included. While studies evaluating palbociclib as part of a broader class of CDK4/6 inhibitors were considered, they were only included in the overall summary. They were excluded from the outcome analyses to prevent confusion from mixing data on palbociclib usage and outcomes with other CDK4/6 inhibitors. The full eligibility criteria are provided in Supplementary Table S1.
Data Extraction and Analysis
Data extraction was conducted in Covidence using a custom template which included:
-
Covidence identification number, study identifier, title and year of publication.
-
Publication type (full-text, congress abstract or poster, erratum).
-
Geographical scope (single-country or cross-country collaborative study).
-
Target countries.
-
Target regions (Africa, Emerging Asia, Latin America, Middle East, Russia, Turkey).
-
Patient population (premenopausal women, postmenopausal women, men, not specified).
-
Treatment (palbociclib only or CDK4/6 inhibitors).
-
Outcomes (clinical [effectiveness and safety], PRO or QoL).
-
Start and end dates.
-
Number of participating centers.
-
Number of participants from target countries.
-
Effectiveness results in first-line and second-line settings.
-
Safety results.
Data were extracted by one reviewer while a second reviewer examined a random sample of extractions to ensure accuracy.
All analyses were performed using Microsoft Excel (Redmond, WA, USA).
Ethical Approval
This research involved only secondary use of data without any patient identifier(s). The article does not contain any new studies with human or animal participants performed by any of the authors. Hence, no ethics approval was necessary.
Results
Literature Search and Screening
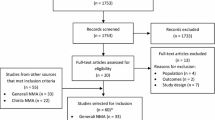
The literature search identified a total of 1699 records (Fig. 1). After excluding 216 duplicates, 1485 records were eligible for phase 1 screening. In this phase, 163 relevant records were identified, while 1322 records were excluded. During phase 2, 49 relevant records were identified, while 114 records were excluded. Most publications excluded during Phase 2 either reported on populations recruited exclusively outside the geographical scope of this review (n = 71) or it was not possible to confirm participation of patients from the target countries (n = 21).
PRISMA diagram. During phase 1 screening, the titles and abstracts of all identified publications were reviewed against the eligibility criteria. Publications that met the eligibility criteria during phase 1 screening proceeded to phase 2 screening. During phase 2 screening, the full text of candidate publications was reviewed, and studies that met the eligibility criteria were included. aWaller (2019) [48]. bKraus (2022) [16], Mycock (2022) [84]. PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses
After phase 2 had been completed, three additional publications were excluded during the data extraction process (duplicate: 1, results for target countries not reported: 2; Fig. 1). Therefore, a total of 46 publications were included in the analysis.
Characteristics of Included Publications
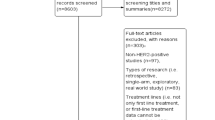
Of the records included in the analysis, 23 (50.0%) were conference abstracts or posters, 22 (47.8%) were full-text publications, and 1 (2.2%) record was an erratum (Supplementary Table S2) (Fig. 2).
Publications included in the analysis by type (n = 46). Congress presentations accounted for the largest proportion of publications included in this review (n = 23, 50.0%) followed closely by full-text publications (n = 22, 47.8%). One publication was an erratum to a full-text publication describing a study conducted in Latin America [85]. Most publications described studies conducted in countries of Emerging Asia (n = 14, 30.4%) and Latin America (n = 14, 30.4%) followed by Turkey (n = 12, 26.1%), the Middle East (n = 6, 10.9%) and Russia (n = 1, 2.2%). None of the publications identified in this scoping review described studies conducted in Africa. Publications from countries of Emerging Asia constituted the largest proportion of congress presentations (34.8%) followed by publications from Latin America (30.4%). Turkey accounted for the largest proportion of full-text publications (36.4%) followed by countries of Emerging Asia and Latin America (27.3% for both). The single publication from Russia included in this review was a congress abstract
Characteristics of Included Studies
The publications included in the analysis reported on 42 individual studies. Three publications reported on the Argentinian cohort of the IRIS study [18, 47, 48]. Two publications reported on the same study conducted in Lebanon [49, 50]. Of note, two publications reported data from two cohorts of patients from Argentina which likely overlapped [51, 52].
Of the 42 individual studies, 38 (90.5%) were conducted in patients recruited from a single target country, while 4 (9.5%) were cross-country collaborations that included patients from one or more target countries (Table 1).
Most studies included both pre- and postmenopausal women (n = 13, 31.0%) followed by studies conducted in pre- and postmenopausal women and in men (n = 6, 14.3%), postmenopausal women only (n = 4, 9.5%), premenopausal women only (n = 1, 2.4%) and men only (n = 1, 2.4%). In addition, one study was conducted in both men and women but did not report the women’s menopausal status (n = 1, 2.4%). A large proportion of studies did not specify the menopausal status of their participants (n = 16, 38.1%).
Slightly less than half of the studies focused on palbociclib in combination with AI or fulvestrant (19 studies, 45.2%). The majority of studies focused on both the first- and second-line treatment experience (n = 24, 57.1%) followed by studies focused on first-line treatment (n = 6, 14.3%) and second-line treatment (n = 5, 11.9%). Line of treatment was not specified in seven studies (n = 16.7%).
Study duration information was available for 35 studies (83.3%), with a median duration of 3.9 (IQR: 2.9 to 4.5) years and a range of 0.3 to 6.0 years (Supplementary Figure S2). Information on the number of participating centers was reported in 28 studies (66.7%), with a median of 2.0 (IQR: 1.0 to 3.0) centers per study and a range of 1 to 46. All studies (n = 42, 100.0%) provided participant numbers, totaling 5624 individuals across the studies. The median study size was 103.5 patients (IQR: 56.8 to 158.8), ranging from 16 to 600. Most included studies reported the number of participants who received palbociclib (n = 37, 88.1%). A total of 3653 participants received palbociclib in the course of the included studies.
Outcomes
Most publications reported only clinical outcomes (n = 44, 95.7%) (Supplementary Table S3, Table 2). One publication (2.2%) reported PRO and one (2.2%) reported both clinical outcomes and PRO.
Effectiveness Outcomes
To evaluate the performance of the combination of palbociclib or other CDK4/6 inhibitors with ET in real-world conditions in countries outside of Western regions, effectiveness outcomes reported in full-text publications are summarized in Table 3. Seven full-text publications reported PFS, one publication reported OS and four publications reported ORR in patients who received palbociclib as first-line therapy. In these studies, the median PFS with palbociclib plus ET ranged from 20.2 to 36.7 months. The median OS with palbociclib plus ET as first-line treatment was 39.9 months, reported only in one publication. The ORR ranged from 45.3% to 80.0%. In second- or later-line endocrine therapy, six, three and four full-text publications reported PFS, OS and ORR with palbociclib plus ET, respectively. The median PFS reported with palbociclib plus ET in these conditions ranged from 7.0 to 24.2 months, the median OS ranged from 11.0 to 19.6 months, and the ORR ranged from 13.9% to 47.9%. Lastly, several full-text publications reported effectiveness outcomes with palbociclib plus ET without specifying line of treatment or for various lines of treatment collectively, including five publications that reported PFS and four publications that reported ORR. None of these publications reported median OS. In this group of publications, the median PFS with palbociclib ranged from 13.0 months to 29.6 months when used in combination with ET and the ORR ranged from 45.8% to 63.3%. Of note, a single study in this group reported efficacy outcomes according to the type of ET [53]. The median PFS was 29.6 months with palbociclib plus AI and 32.7 months with palbociclib plus fulvestrant. An additional relevant study was identified after the systematic search cutoff date. This expanded access trial involved 130 postmenopausal women from Latin America (Argentina, Brazil, Colombia and Mexico), with 54.6% having undergone ≥ 2 lines of systemic therapies prior to palbociclib [54]. The ORR was 24.8%, and the median treatment duration was 10.6 months.
Effectiveness outcomes in conference abstracts and posters are summarized in Supplementary Table S4. In these publications, first-line palbociclib plus ET was associated with the median PFS of between 18.0 and 30.3 months and an ORR of between 41.7% to 71.0%, while second- or later-line palbociclib plus ET was associated with a median PFS of 13.0 to 14.0 months and an ORR of 52.0%.
Safety Outcomes
Safety outcomes commonly reported in full-text publications are summarized in Table 4. In full-text publications, the incidence of any-grade AEs in patients who received palbociclib plus ET ranged from 80.0% to 91.1%, the incidence of grade ≥ 3 AEs ranged from 10.0% to 72.1%, the incidence of any-grade neutropenia ranged from 76.9% to 82.0%, and the incidence of grade ≥ 3 neutropenia ranged from 20.0% to 65.4%. In addition, between 0 and 7.6% of patients had febrile neutropenia. Infections occurred in 1.3–44.3% of patients. Similar variability was observed regarding treatment modifications, with interruptions, dose modifications and discontinuations occurring in 14.9–64.0%, 14.0–53.3% and 0–4.8% of patients, respectively.
Safety outcomes reported in conference abstracts or posters (Supplementary Table S5) were broadly consistent with those reported in full-text publications.
Furthermore, the safety-related results were consistent in the expanded access trial from Latin America that we retrieved via a manual search (published after the date of our systematic search) [54]. Neutropenia was the most common treatment-related AE reported in 70.0% patients in this study, and 22.3% of patients required dose reduction of palbociclib due to AEs.
PRO
Two publications included in this analysis described PRO. One was a cross-sectional survey conducted in patients who received palbociclib in combination with an AI or fulvestrant in several countries [55]. This study included 51 patients from Argentina and used 16 questions from the Cancer Therapy Satisfaction Questionnaire (CTSQ) to evaluate expectations of therapy, feelings about side effects and satisfaction with therapy [55]. The results showed that 76.8% of patients believed that palbociclib therapy would help them return to normal life, 92.2% reported that side effects were as they expected or better, and 93.5% of patients stated that they would take palbociclib again [55]. The second study was conducted in 80 patients who received CDK4/6 inhibitors in combination with ET for advanced breast cancer in Turkey [56]. The study used Pittsburgh Sleep Quality Index (PSQI) to evaluate sleep quality and duration as well as daytime dysfunction [56]. In this study, 68.8% of patients had poor sleep quality. There were no differences in PSQI scores between patients on palbociclib and ribociclib. However, sleep quality was worse in patients who received letrozole than in patients who received fulvestrant (p = 0.042) [56].
The retrieved list of citations included a protocol for the RWD MADELINE Asia study, which was conducted in four Asian countries: Taiwan, India, Malaysia and Hong Kong [57]. Although this citation was ineligible for inclusion as a study protocol, a manual search after data analysis revealed that the results of the MADELINE Asia study had been presented at the Asia Pacific Breast Cancer Summit in 2023 [58]. The study enrolled patients with HR-positive, HER2-negative advanced breast cancer who were treated with palbociclib and an AI. The study used the 12-item Short-Form Health Survey (SF-12) and the 10-item Center for Epidemiological Studies-Depression Scale (CES-D-10) to evaluate the effects of anticancer therapy over a 6-month period. Key findings of the MADELINE Asia study include stable general health, low levels of pain and fatigue and overall high treatment satisfaction, with more than half of the patients being “satisfied” or “very satisfied” throughout the study period. Most patients rated their mood as neutral or positive, with ≤ 6% reporting negative mood across all treatment cycles [58].
Discussion
The present scoping review evaluated the RWD on the use of palbociclib, either in combination with an AI as first-line therapy or in combination with fulvestrant following progression on ET, in patients with HR-positive, HER2-negative advanced or metastatic breast cancer in countries outside of Western regions. The literature search identified a total of 46 publications, including 23 conference abstracts or posters and 22 full-text publications. These publications described 42 involving a total of 5624 patients. Emerging Asia, Latin America and Turkey accounted for the majority of publications, while the Middle East and Russia were underrepresented, and no studies from Africa were identified.
There were notable differences in the quality of reporting between conference presentations and full-text publications. As a result, effectiveness and safety outcomes were summarized separately to ensure a more rigorous and dependable synthesis of the reviewed literature.
Overall, palbociclib plus ET demonstrated effectiveness in real-world settings similar to the efficacy reported in the PALOMA trials [59,60,61]. In full-text publications, first-line palbociclib plus ET was associated with a median PFS of between 20.2 to 36.7 months and a median OS of 39.9 months in the one publication that reported it [53, 62, 63]. The ORR ranged from 45.3% to 80.0% in first-line setting. In the second- and later-line setting, palbociclib plus ET was associated with a median PFS of between 7.0 and 24.2 months, a median OS of between 11.0 and 19.6 months, and an ORR of between 13.9% and 47.9% [53, 64, 65]. Of note, in second- or later-line therapy, the upper boundary of the PFS range was higher than the upper boundary of the OS range. This was due to the fact that some of the publications reported PFS but not OS. In the publication that reported a median PFS of 24.2 months with second- or later-line palbociclib, median OS in this setting was not reached [53]. All publications that reported both PFS and OS reported a lower value for the former than for the latter. Furthermore, a PFS of 7.0 months and OS of 11.0 months were reported in a population of heavily pretreated patients, all of whom received ≥ 3 previous lines of systemic therapy [64].
In RCTs versus AI alone, palbociclib plus AI was associated with a median PFS of 24.8 months [8]. In RCTs versus fulvestrant alone, palbociclib plus fulvestrant was associated with a median PFS of 9.5 months [8]. The median OS was 34.9 months in RCTs of palbociclib plus ET conducted in patients with advanced breast cancer [8]. In the present analysis, comparisons between palbociclib and ribociclib in RWD were mixed: studies from Turkey found no significant differences in PFS, while a Brazilian study reported longer median PFS for ribociclib plus ET, though this was attributed to treatment group imbalances [66,67,68]. Evaluations of CDK4/6 inhibitors by body mass index also showed mixed results, with one Turkish study reporting longer PFS in overweight patients and another finding no significant differences [69, 70]. HER2-low phenotype was linked to shorter PFS in a Hong Kong study, but no significant differences were found in a Turkish study [67, 71]. Additionally, patients with PIK3CA mutations or pathogenic variants of gBRCA1/2, ATM and CHEK2 genes had shorter PFS and OS when treated with CDK4/6 inhibitors, highlighting the impact of genetic mutations on treatment outcomes [72, 73].
Safety profiles from RWD were generally consistent with clinical trial data. The incidence of AEs of any grade with palbociclib plus ET ranged from 39.2% to 94.4% (grade ≥ 3 AEs: 10.0–72.1%). The incidence of neutropenia, an AE often associated with palbociclib, ranged from 12.8% to > 95.0% (grade ≥ 3: 15.4–74.5%) [8]. The incidence of febrile neutropenia ranged from 0% to 7.6%. The authors of the study that reported the latter value noted that of the eight patients who had febrile neutropenia, six were > 65 years old and most had bone involvement, were heavily pretreated and had received palliative radiation therapy previously [53].
One relevant publication was identified after the systematic search had been completed. This was an expanded access study that was conducted in 130 postmenopausal women from Argentina, Brazil, Colombia and Mexico [54]. Most participants (90.0%) received at least one prior systemic therapy in either the adjuvant or metastatic setting, and slightly over half (54.6%) received ≥ 2 prior lines of systemic therapy. After participants received palbociclib plus letrozole for a median of 10.6 months, the ORR was 24.8%. Treatment duration was relatively short because palbociclib was approved during the study, leading to its termination. The most common AEs were neutropenia (70.0%), leukopenia (34.6%), anemia (33.8%), decreased neutrophil count (27.7%) and thrombocytopenia (24.6%). In addition, AEs resulted in the discontinuation of palbociclib in 12.3% of participants and in palbociclib dose reductions in 22.3% of participants [54]. Therefore, the safety results of this expanded access study were consistent with those of the present analysis.
This scoping review had a number of limitations. First, the search was limited to English-language publications; as a result, relevant publications in other languages could have been missed. Second, while care was taken to identify publications reporting on the same study, it is possible that some of the studies were conducted in overlapping patient cohorts. Third, our review relied on the definitions of lines of therapy as provided in the source papers, which may have introduced heterogeneity. Different studies may have varying criteria for defining first and subsequent lines of therapy, which could affect the comparability of outcomes. This potential variability should be considered when interpreting the results [74]. Furthermore, the results of the included studies could have been influenced by other regional differences, such as variations in healthcare systems. Due to this variability, meaningful comparisons between included publications and with published literature are difficult. The quality of reporting was also poor in some of the included publications, and the variation in reported outcomes could have made comparisons with RCTs difficult. However, only full-text publications, which were characterized by a higher standard of reporting, were considered for this purpose. Therefore, it is hoped that the findings of this review will improve our understating of the real-world effectiveness and safety of palbociclib in the context of PALOMA trials.
Conclusions
The findings of this scoping review underscore the critical role of RWD in complementing RCTs, particularly in the context of breast cancer treatment with palbociclib in countries outside of Western regions. RWD provides invaluable insights into the practical application and implications of therapies in diverse, real-world settings, addressing gaps often left by RCTs which may not fully represent the spectrum of patient populations and clinical scenarios encountered in everyday practice.
Specifically, our analysis revealed that the real-world effectiveness and safety of palbociclib plus ET in populations from countries outside of Western regions are broadly consistent with the outcomes observed in the PALOMA trials. This finding is significant, as it suggests that the benefits of palbociclib observed under the controlled conditions of RCTs extend to more varied clinical environments and patient demographics in countries outside of Western regions. Such data are especially vital for regions such as Latin America and Emerging Asia, where RCTs are less frequently conducted. It highlights the relevance of palbociclib plus ET as a valuable treatment option for HR-positive, HER2-negative advanced or metastatic breast cancer in these regions.
The findings also highlight a number of gaps in published RWD from some regions/ countries. Few publications described the use of palbociclib in the Middle East and Russia and none in Africa. Furthermore, the majority of publications focused on clinical outcomes, while only two publications reported PROs. These findings highlight the need for additional real-world studies to be conducted. Additionally, there is a need for enhancement in the quality and accuracy of the data reported in abstracts and posters.
Data Availability
All supplementary tables and figures generated during this study are included in this published article as supplementary information files. Other datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. 2019;173(1):37–48.
Palmieri C, Owide J, Fryer K. Estimated prevalence of metastatic breast cancer in England, 2016–2021. JAMA Netw Open. 2022;5(12): e2248069.
Sedeta ET, Jobre B, Avezbakiyev B. Breast cancer: global patterns of incidence, mortality, and trends. J Clin Oncol. 2023;41(16_suppl):10528.
Lim YX, Lim ZL, Ho PJ, Li J. Breast cancer in asia: incidence, mortality, early detection, mammography programs, and risk-based screening initiatives. Cancers (Basel). 2022;14(17):4218.
Rajappa S, Singh M, Uehara R, Schachterle SE, Setia S. Cancer incidence and mortality trends in Asia based on regions and human development index levels: an analyses from GLOBOCAN 2020. Curr Med Res Opin. 2023;39(8):1127–37.
O’Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13(7):417–30.
Braal CL, Jongbloed EM, Wilting SM, Mathijssen RHJ, Koolen SLW, Jager A. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: similarities and differences. Drugs. 2021;81(3):317–31.
Battisti NML, Ring A. CDK4/6 inhibition in HER2-positive breast cancer. Lancet Oncol. 2020;21(6):734–5.
National Comprehensive Cancer Network. Breast Cancer 2024 [updated 3 July 2024. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Im SA, Gennari A, Park YH, Kim JH, Jiang ZF, Gupta S, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, staging and treatment of patients with metastatic breast cancer. ESMO Open. 2023;8(3): 101541.
Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res BCR. 2018;20(1):37.
Beachler DC, de Luise C, Jamal-Allial A, Yin R, Taylor DH, Suzuki A, et al. Real-world safety of palbociclib in breast cancer patients in the United States: a new user cohort study. BMC Cancer. 2021;21(1):97.
DeMichele A, Cristofanilli M, Brufsky A, Liu X, Mardekian J, McRoy L, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in US real-world clinical practice. Breast Cancer Res BCR. 2021;23(1):37.
Cardoso Borges F, Alves da Costa F, Ramos A, Ramos C, Bernardo C, Brito C, et al. Real-world effectiveness of palbociclib plus fulvestrant in advanced breast cancer: results from a population-based cohort study. Breast. 2022;62:135–43.
Kraus AL, Yu-Kite M, Mardekian J, Cotter MJ, Kim S, Decembrino J, et al. Real-world data of palbociclib in combination with endocrine therapy for the treatment of metastatic breast cancer in men. Clin Pharmacol Ther. 2022;111(1):302–9.
Mycock K, Zhan L, Hart K, Taylor-Stokes G, Milligan G, Atkinson C, et al. Real-world treatment of patients with palbociclib for HR+/HER2-advanced/metastatic breast cancer: the Europe IRIS study. Future Oncol (London, England). 2022;18(3):349–62.
Mycock K, Hanson KA, Taylor-Stokes G, Milligan G, Atkinson C, Mitra D, et al. Real-world treatment patterns and clinical outcomes associated with palbociclib combination therapy: a multinational, pooled analysis from the ibrance real world insights study. Clin Ther. 2022;44(12):1588–601.
Tripathy D, Rocque G, Blum JL, Karuturi MS, McCune S, Kurian S, et al. 251P Real-world clinical outcomes of palbociclib plus endocrine therapy (ET) in hormone receptor–positive advanced breast cancer: results from the POLARIS trial. Ann Oncol. 2022;33:S651–2.
DeMichele A, Robert N, Chen C, Kim S, Zhang Z, Lu DR, et al. Real-world tumor response of palbociclib in combination with an aromatase inhibitor as first-line therapy in pre/perimenopausal women with metastatic breast cancer. Target Oncol. 2023;18(4):543–58.
Lok SW, Tung I, Anton A, Baron-Hay SE, De Boer RH, Boyle FM, et al. Real world evidence of systemic therapy in hormone receptor positive advanced breast cancer (HR+ ABC) in Australia: ARORA Registry. J Clin Oncol. 2023;41(16):e13054.
Palmieri C, Musson A, Harper-Wynne C, Wheatley D, Bertelli G, Macpherson IR, et al. A real-world study of the first use of palbociclib for the treatment of advanced breast cancer within the UK National Health Service as part of the novel Ibrance® Patient Program. Br J Cancer. 2023;129:159–60.
US Food and Drug Administration. Enhancing the diversity of clinical trial populations—eligibility criteria, enrollment practices, and trial designs guidance for industry 2020. https://www.fda.gov/media/127712/download.
Brufsky A, Gallagher C. P-REALITY X: a real-world analysis of palbociclib plus an aromatase inhibitor in HR+/HER2− metastatic breast cancer-a podcast. Target Oncol. 2023;18(3):321–6.
Ranganathan K, Singh P, Wilkins EG, Hamill JB, Aliu O, Newman L, et al. Abstract 03: the global macroeconomic burden of breast cancer: implications for oncologic and reconstructive surgery. Plast Reconstr Surg Glob Open. 2018;6(4 Suppl):2–3.
Ji P, Gong Y, Jin ML, Hu X, Di GH, Shao ZM. The burden and trends of breast cancer from 1990 to 2017 at the global, regional, and national levels: results from the global burden of disease study 2017. Front Oncol. 2020;10:650.
Shau WY, Setia S, Shinde S, Santoso H, Furtner D. Generating fit-for-purpose real-world evidence in Asia: how far are we from closing the gaps? Perspect Clin Res. 2023;14(3):108–13.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Ogden K, Thompson JC, Halfpenny NJ, Scott DA. A conceptual search filter to identify real-world evidence. Value in Health. 2015;18(7):A728.
Charles D, Shanley J, Temple SN, Rattu A, Khaleva E, Roberts G. Real-world efficacy of treatment with benralizumab, dupilumab, mepolizumab and reslizumab for severe asthma: a systematic review and meta-analysis. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2022;52(5):616–27.
Deitelzweig S, Bergrath E, di Fusco M, Kang A, Savone M, Cappelleri JC, et al. Real-world evidence comparing oral anticoagulants in non-valvular atrial fibrillation: a systematic review and network meta-analysis. Future Cardiol. 2022;18(5):393–405.
Shau WY, Setia S, Shinde SP, Santoso H, Furtner D. Contemporary databases in real-world studies regarding the diverse health care systems of India, Thailand, and Taiwan: protocol for a scoping review. JMIR Res Protoc. 2022;11(12): e43741.
Adis International Ltd. Palbociclib—Chia Tai Tianqing Pharmaceutical 2023 [updated 05 May 2023]. https://adisinsight.springer.com/drugs/800051802.
Adis International Ltd. Palbociclib—onyx pharmaceuticals/Pfizer 2023 [updated 02 Jul 2023]. https://adisinsight.springer.com/drugs/800020668.
Shvili J. The Western World 2021 [updated 26 April 2021. https://www.worldatlas.com/articles/list-of-western-countries.html.
Wikimedia Foundation, Inc. Western world 2023 [updated 14 August 2023. https://en.wikipedia.org/wiki/Western_world.
Odan N, Kikawa Y, Matsumoto H, Minohata J, Suwa H, Hashimoto T, et al. Real-world outcomes of treating advanced breast cancer patients with palbociclib: a multicenter retrospective cohort study in Japan-The KBCOG-14 study. Breast Cancer Basic Cli Res. 2020;14:1178223420983843.
Shangguan CF, Jiang M, Yang C, Lou GY, Li YT, Qu Q. Clinical efficacy of palbociclib-based therapy in women with HR+/HER2− metastatic breast cancer in the real-world setting for Chinese women: a comparison with the IRIS study. Eur Rev Med Pharmacol Sci. 2021;25(19):6138–48.
Lee J, Park HS, Won HS, Yang JH, Lee HY, Woo IS, et al. Real-world clinical data of palbociclib in asian metastatic breast cancer patients: experiences from eight institutions. Cancer Res Treat. 2021;53(2):409–23.
Shen L, Zhou J, Chen Y, Ding J, Wei H, Liu J, et al. Treatment patterns, effectiveness, and patient-reported outcomes of palbociclib therapy in Chinese patients with advanced breast cancer: a multicenter ambispective real-world study. Cancer Med. 2022;11(22):4157–68.
World Population Review. Transcontinental countries 2023. 2023. https://worldpopulationreview.com/country-rankings/transcontinental-countries.
Ali Jadoo SA, Aljunid SM, Sulku SN, Nur AM. Turkish health system reform from the people’s perspective: a cross sectional study. BMC Health Serv Res. 2014;14:30.
Bener A, Alayoglu N, Çatan F, Torun P, Yilmaz ES. Health services management in Turkey: failure or success? Int J Prev Med. 2019;10:30.
Irwin-Hunt A. Turkey pushes its life sciences to next level 2021 [updated 13 December 2021. https://www.fdiintelligence.com/content/feature/turkey-pushes-its-life-sciences-to-next-level-80446.
Shishkin S, Sheiman I, Vlassov V, Potapchik E, Sazhina S. Structural changes in the Russian health care system: do they match European trends? Heal Econ Rev. 2022;12(1):29.
Barrios C, de Lima LG, Yusof MM, Rubagumya F, Rutkowski P, Sengar M. Barriers in access to oncology drugs—a global crisis. Nat Rev Clin Oncol. 2023;20(1):7–15.
Taylor-Stokes G, Waller J, Mitra D, Gibson K, Milligan G, Iyer S. Real world treatment patterns and clinical outcomes associated with palbociclib combination therapy in Argentina: results from the IRIS study. J Clin Oncol. 2018;36(15):e13026.
Waller J, Mitra D, Mycock K, Taylor-Stokes G, Milligan G, Zhan L, et al. Real-world treatment patterns and clinical outcomes in patients receiving palbociclib for hormone receptor–positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer in Argentina: the IRIS study. J Glob Oncol. 2019;5:Jgo800239.
Nasr F, Ghoche A, Diab S, Nasr L. Real-world survival data of palbociclib in advanced and metastatic breast cancer: a multicenter experience in lebanon. Breast. 2019;48:S53.
Nasr L, Ghoche A, Diab S, Nasr F. Real-world survival data of palbociclib in advanced and metastatic breast cancer: a multicenter experience in Lebanon. J Clin Oncol. 2020;38(15):e13054.
Mainella A, Vigo S, Marmissolle F, Price P, Sansano M, Luján ML, et al. Palbociclib in the treatment of metastatic breast cancer. Institutional experience. Int J Gynecol Cancer. 2019;29:624.
Mainella A, Marmissolle F, Vigo S, Price P, Sansano M, Lujan ML, et al. Palbociclib in the daily clinical use: real experience in metastatic breast cancer in our institution. Int J Gynecol Cancer. 2020;30(SUPPL 3):A98–9.
Petracci F, Abuin GG, Pini A, Chacón M. RENATA study-Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer-real-world use. Ecancermedicalscience. 2020;14:1058.
Fein L, Lazaretti N, Chuken YL, Benfield J, Mano MS, Lobaton J, et al. Expanded access study of palbociclib plus letrozole for postmenopausal women with HR+/HER2− advanced breast cancer in Latin America for whom letrozole therapy is deemed appropriate. Clin Drug Investig. 2023;43(9):699–706.
Darden C, Mitra D, McSorley D, Davis K, Band J, Iyer S. Treatment satisfaction in women receiving palbociclib combination therapies for advanced/metastatic breast cancer. Future Oncol. 2019;15(2):141–50.
Dülgar Ö, Ferik S, Ay S, Bayram E, Şakalar T. Sleep quality analysis in metastatic breast cancer patients receiving cyclin-dependent kinase 4–6 inhibitor. J Oncol Sci. 2022;8(3):113–8.
Tai AYP, Singh M, Binko J, Lilly K, Chang S, Bowles S, et al. 69TiP MADELINE Asia: a mobile app-based prospective observational study of patient reported outcomes in advanced breast cancer in Asia. Ann Oncol. 2020;31:S1269.
Lee, K-T, Liu C-Y, Kwong HW, Ang SF, Rauthan A, Singh M, et al. Madeline Asia: a smartphone-application–based, prospective, observational study of patient-reported outcomes and quality of life for people with HR+/HER2– advanced breast cancer receiving palbociclib + aromatase inhibitor in Asia. In: Asia-Pacific breast cancer summit (APBCS), Singapore; 2023.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36.
Cristofanilli M, Rugo HS, Im SA, Slamon DJ, Harbeck N, Bondarenko I, et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase III randomized study. Clin Cancer Res. 2022;28(16):3433–42.
Xu B, Hu X, Li W, Sun T, Shen K, Wang S, et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: primary results from PALOMA-4. Eur J Cancer. 2022;175:236–45.
Agrawal C, Goyal P, Agarwal A, Tripathi R, Dodagoudar C, Baghmar S, et al. Multicentric real world evidence with palbociclib in hormone positive HER2 negative metastatic breast cancer in Indian population. Sci Rep. 2021;11(1):16236.
Ganguly S, Mukherjee N, Mandal S, Roy S, Agarwal S, Biswas B, et al. Efficacy of cyclin-dependent kinase 4/6 inhibitors in patients with metastatic hormone positive breast cancer: a single institutional study from India. Ecancermedicalscience. 2022;16:1450.
Demir A, Mandel NM, Paydas S, Demir G, Er Ö, Turhal NS, et al. Efficacy of palbociclib and endocrine treatment in heavily pretreated hormone receptor-positive/HER2-negative advanced breast cancer: retrospective multicenter trial. Balkan Med J. 2020;37(2):104–7.
Al-Foheidi MH, Albeshri AM, Moamenkahan SN, Abdullah AM, Abualola MS, Alharbi MH, et al. Combination of palbociclib with adjuvant endocrine therapy for treatment of hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer: an experience at two cancer centers in Saudi Arabia. Mol Clin Oncol. 2022;17(1):119.
Kahraman S, Erul E, Seyyar M, Gumusay O, Bayram E, Demirel BC, et al. Treatment efficacy of ribociclib or palbociclib plus letrozole in hormone receptor-positive/HER2-negative metastatic breast cancer. Future Oncol (London, England). 2023;19(10):727–36.
Kahraman S, Seyyar M, Sahin E, Cabuk D, Gumusay O, Basaran G, et al. Impact of HER2 expression levels on survival in patients with hormone receptor positive and HER2 negative advanced breast cancer and treated with ribociclib and palbociclib in combination with endocrine therapy: a real-life data, Turkish Oncology Group study. ESMO Open. 2023;8(1):101440.
Queiroz MM, Sacardo KP, Ribeiro MF, Gadotti LL, Saddi R, Oliveira LJC, et al. Real-world treatment outcomes in HR+ HER2− metastatic breast cancer patients treated with CDK4/6 inhibitors: results from a reference center in Brazil. Cancer Treat Res Commun. 2023;35: 100683.
Artac M, Cağlayan D, Koçak MZ, Geredeli C, Tatli AM, Sezgin Goksu S, et al. The impact of body mass index (BMI) on the progression-free survival of CDK4/6 inhibitors in metastatic breast cancer patients (MBC). Ann Oncol. 2022;33:S644–5.
Çaǧlayan D, Kocak MZ, Geredeli C, Tatli AM, Eryõlmaz MK, Göksu SS, et al. The effect of BMI on the outcomes of CDK 4/6 inhibitor therapy in HR-positive metastatic breast cancer patients. J Clin Oncol. 2022;40(16):e13010.
Bao KKH, Sutanto L, Tse SSW, Man Cheung K, Chan JCH. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open. 2021;4(11):e2133132.
Pavithran K, Jayamohanan H, Jose WM, Soman S, Vijaykumar DK, Ariyannur PS. PI3K mutation is associated with reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer. Ann Oncol. 2021;32:S471.
Bruno L, Ostinelli A, Waisberg F, Enrico D, Ponce C, Rivero S, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair-related genes. JCO Precis Oncol. 2022. https://doi.org/10.1200/PO.21.00140.
Saini KS, Twelves C. Determining lines of therapy in patients with solid cancers: a proposed new systematic and comprehensive framework. Br J Cancer. 2021;125(2):155–63.
Lakkavalli RK, Pehalajani JK, Tirumala V, Babu GK, Loknatha D, Jacob LA, et al. Metastatic hormone receptor-positive breast cancer in CDK 4/6 era: an outcome audit. J Cancer Res Ther. 2021;17(4):994–7.
Low JL, Lim E, Bharwani L, Wong A, Wong K, Ow S, et al. Real-world outcomes from use of CDK4/6 inhibitors in the management of advanced/metastatic breast cancer in Asia. Ther Adv Med Oncol. 2022. https://doi.org/10.1177/17588359221139678.
Rath S, Elamarthi P, Parab P, Gulia S, Nandhana R, Mokal S, et al. Efficacy and safety of palbociclib and ribociclib in patients with estrogen and/or progesterone receptor positive, HER2 receptor negative metastatic breast cancer in routine clinical practice. PLoS ONE. 2021;16(7 July):e0253722.
Yıldırım H, Mutlu E, Chalabiyev E, Özen M, Keskinkılıç M, Ön S, et al. Clinical outcomes of cyclin-dependent kinase 4–6 (CDK 4–6) inhibitors in patients with male breast cancer: a multicenter study. Breast. 2022;66:85–8.
Çağlayan D, Koçak MZ, Geredeli Ç, Tatlı AM, Göksu SS, Eryılmaz MK, et al. The effect of concomitant use of proton pump inhibitors with CDK 4/6 inhibitors on survival in metastatic breast cancer. Eur J Clin Pharmacol. 2023;79(2):243–8.
Eser K, Önder AH, Sezer E, Çil T, İnal A, Öztürk B, et al. Proton pump inhibitors may reduce the efficacy of ribociclib and palbociclib in metastatic breast cancer patients based on an observational study. BMC Cancer. 2022. https://doi.org/10.1186/s12885-022-09624-y.
Keskinkilic M, Semiz HS, Polat G, Arayici ME, Yavuzsen T, Oztop I. The prognostic indicator in breast cancer treated with CDK4/6 inhibitors: the prognostic nutritional index. Future Oncol. 2023;19(7):517–29.
Odabas H, Dogan A, Ozcelik M, Yildirim S, Ozkerim U, Turan N, et al. Does proton pump inhibitors decrease the efficacy of palbociclib and ribociclib in patients with metastatic breast cancer? Medicina (Kaunas). 2023;59(3):557.
Loi S, Karapetis CS, McCarthy N, Oakman C, Redfern A, White M, et al. Palbociclib plus letrozole as treatment for postmenopausal women with hormone receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer for whom letrozole therapy is deemed appropriate: an expanded access study in Australia and India. Asia Pac J Clin Oncol. 2022;18(6):560–9.
Mycock K, Hanson K, Taylor-Stokes G, Milligan G, Atkinson C, Mitra D, et al. POSB400 real world treatment patterns and clinical outcomes associated with palbociclib combination therapy across Europe, North and South America, and Asia: a pooled analysis from the IRIS study. Value Health. 2022;25(1):S259.
Petracci F, Abuin GG, Pini A, Chacón M. Erratum: RENATA study—Latin American prospective experience: clinical outcome of patients treated with palbociclib in hormone receptor-positive metastatic breast cancer—real-world use (ecancermedicalscience (2020) 14 (1058) https://doi.org/10.3332/ecancer.2020.1058). Ecancermedicalscience. 2020;14.
Medical Writing, Editorial and Other Assistance
The authors sincerely appreciate the assistance provided by Dr. Sajita Setia and Georgii Filatov from Transform Medical Communications, New Zealand, for Phase 1 and Phase 1 screening, data extraction, analysis and medical writing.
Funding
This study, including the journal’s Rapid Service fee, was funded by Pfizer. In addition, Pfizer provided funding for the medical writing and editorial assistance associated with this manuscript.
Author information
Authors and Affiliations
Contributions
Amit Rauthan, Ankita Jain, Manmohan Singh and Mehmet Ali Nahit Sendur made substantial contributions to the conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Ankita Jain and Manmohan Singh are employees of Pfizer. They report no other conflicts of interest in this work. The results and discussions in this article do not represent or reflect in any way the official policy or position of the current or previous employers of the authors. Amit Rauthan and Mehmet Ali Nahit Sendur have no conflicts of interest to declare.
Ethical Approval
This research involved only secondary use of data without any patient identifier(s). The article does not contain any new studies with human or animal participants performed by any of the authors. Hence, no ethics approval was necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rauthan, A., Jain, A., Singh, M. et al. Palbociclib in HR-Positive, HER2-Negative Advanced/Metastatic Breast Cancer: A Systematic Scoping Review of Real-World Evidence from Countries Outside of Western Regions that Are Underrepresented in Clinical Trials. Oncol Ther 12, 395–418 (2024). https://doi.org/10.1007/s40487-024-00295-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-024-00295-2