Abstract
Introduction
Approximately half of patients with non-small cell lung cancer (NSCLC) present with early-stage disease at diagnosis. Real-world outcomes data are limited for this population but are of interest given recent and impending results from trials evaluating epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) and immunotherapies in neoadjuvant, adjuvant, and perioperative settings.
Methods
A retrospective, longitudinal, population-level study was conducted in patients diagnosed with resected stage I–III non-squamous NSCLC in Ontario, Canada, between April 2010 and March 2019. Study outcomes included patient characteristics and median overall survival (mOS), with stratification by disease stage and treatment exposure. Patients receiving EGFR-TKIs (assumed EGFR mutation-positive by proxy) were a key population of interest.
Results
Among 8255 cases, 4881 had stage I, 2124 had stage II, and 1250 had stage III NSCLC at diagnosis. The mean patient age was 68 years; 53.5% were female. In the overall cohort, 19.6% received adjuvant chemotherapy. Receipt of adjuvant chemotherapy was associated with significantly longer mOS than not receiving such therapy: stage II (7.6 [95% confidence interval: 6.5–8.5] vs. 4.4 [4.0–4.9] years) or stage III (4.4 [3.6–5.1] vs. 2.7 [2.3–3.3] years), both p < 0.0001. Patients receiving treatment (EGFR-TKIs and chemotherapy) were assumed to have experienced disease recurrence/relapse; mOS was longer among those receiving an EGFR-TKI than among those receiving chemotherapy (2.3 [1.8–3.0] vs. 1.1 [1.0–1.3] years).
Conclusion
In Ontario, between 2010 and 2019, uptake of adjuvant therapy was low among patients with resected NSCLC, despite such therapy being associated with improved survival. Patients assumed to have recurred/relapsed had markedly reduced mOS, regardless of subsequent therapy, compared with those who did not relapse/recur. Novel peri-adjuvant treatment options are needed to enhance outcomes after lung resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Management of early-stage resected non-small cell lung cancer (NSCLC) is quickly evolving to include new neoadjuvant and adjuvant treatment options. |
It is therefore of interest to understand treatment patterns and clinical outcomes such as survival among patients with resected stage I–III NSCLC who may be eligible for such therapies. |
In this real-world study conducted in Ontario, Canada, use of adjuvant chemotherapy was low between 2010 and 2019, even though such treatment was observed to significantly prolong survival among patients with stage II/III disease. |
Among patients whose disease was assumed to have recurred/relapsed, median overall survival was markedly reduced compared with that of patients whose disease was assumed to not have recurred/relapsed, regardless of subsequent therapy. |
These findings underscore a high need for the newly available and anticipated peri-adjuvant treatment options to improve clinical outcomes in early-stage resected NSCLC. |
Introduction
Lung cancer is the most commonly diagnosed malignancy in Canada, accounting for an estimated 13% of all new cancer cases and 24% of all cancer-related deaths in 2023 [1]. Approximately 31,000 new lung cancer diagnoses occurred across the country in 2023 [1], with 10,639 new cases in the province of Ontario in 2022 [2]. Of these diagnoses, non-small cell lung cancer (NSCLC) was the most common disease subtype, representing an estimated 88% of all cases [3]. In Canada, approximately 45–66% of patients with NSCLC present with early-stage disease (stage I–III) at diagnosis [3], in part because of the recent introduction of screening programs that support early detection [4,5,6].
Among eligible individuals, guideline-recommended treatment of early-stage NSCLC conventionally involves surgical resection (e.g., lobectomy, segmentectomy, or pneumonectomy) followed by adjuvant chemotherapy depending on patient and tumor characteristics [7,8,9]. For some patients, such treatment is associated with greatly prolonged survival that may approach cure [7, 10]. For others, however, the risk of recurrence remains high, ranging from 45 to 76% at 5 years among patients with stage IB through stage III disease [10]. Upon progression to advanced disease stages, the goals of therapy shift from cure to prolongation of survival and maintenance of health-related quality of life [11,12,13]. In this setting, recommended treatment options include systemic therapies such as single-agent or doublet platinum-based chemotherapy, molecularly targeted options, and immunotherapy [14, 15]. Numerous molecular biomarkers are now clinically available, such as gene alterations of the epidermal growth factor receptor (EGFR), the Kristen rat sarcoma viral oncogene homolog gene (KRAS), and anaplastic lymphoma kinase (ALK), as well as the programmed cell death 1 receptor (PD-1) and its ligand (PD-L1) [16, 17].
Given the high rates of disease recurrence in NSCLC and the benefits of novel treatments used in advanced NSCLC settings, the efficacy of these agents is being evaluated in earlier disease stages. One such drug category is epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), which are targeted to patients with certain EGFR mutations (EGFRm). These mutations are observed in 14–22% of Canadian NSCLC cases [18,19,20,21,22,23], with a similar prevalence reported between patients with early-stage versus advanced disease [24, 25]. Initial evaluations of early-generation EGFR-TKIs (i.e., erlotinib and gefinitib) in the adjuvant NSCLC setting showed a limited survival impact and did not lead to changes in clinical practice [26,27,28,29,30,31,32,33]. However, more recently, the third-generation EGFR-TKI osimertinib was associated with favorable outcomes for disease-free survival (DFS) and overall survival (OS) in the phase III ADAURA trial of resected stage IB–IIIA NSCLC [34,35,36]. These results led to osimertinib becoming the first targeted agent approved for adjuvant use in early-stage NSCLC after complete resection, with approval received from Health Canada [37] and multiple other regulatory bodies in 2020 and 2021 [38, 39]. Several other trials of agents used in neoadjuvant and adjuvant settings (e.g., immunotherapies) are ongoing or have now shown similarly positive results, further transforming the management and outcomes of early-stage NSCLC [40,41,42].
With approximately 15 million individuals, the province of Ontario represents 39% of the Canadian population [43]. Recent real-world studies of NSCLC in this region have focused on patients with advanced or metastatic disease, reporting on treatment patterns and survival results [44,45,46,47,48]; others have analyzed much older datasets [49,50,51]. Therefore, there is a paucity of contemporary, real-world, population-level outcomes data for patients with resected early-stage NSCLC. Such data would provide context for the true unmet needs of this patient population in light of growing evidence for the use of novel therapeutics in early-stage patients. Therefore, the objective of the current study was to understand the baseline characteristics and clinical outcomes of patients with resected stage I–III NSCLC living in Ontario, Canada, with a specific focus on those with an EGFRm.
Methods
Study Design
In collaboration with the HOPE Research Centre and the Institute of Clinical Evaluative Sciences (ICES) Data & Analytic Services (DAS), a retrospective, longitudinal evaluation was conducted of real-world, population-level data. Eligible patients included those diagnosed with NSCLC in Ontario, Canada, between April 1, 2010, and March 31, 2019, who had at least 6 months of follow-up after diagnosis. Included patients had a valid Ontario Health Insurance Plan (OHIP) card and met the following inclusion criteria: International Classification of Disease for Oncology, third edition (ICDO3) diagnosis code C34 (locally advanced lung cancer) and relevant morphology codes in the Ontario Cancer Registry (OCR); non-squamous histology; stage I, II, or III NSCLC; and underwent ≥ 1 surgery for lung resection within 180 days of NSCLC diagnosis, as indicated by Canadian Classification of Health Interventions (CCI) codes. Patients were excluded from the study if they did not have a valid ICES (IKN) number, did not have a lung cancer diagnosis in the OCR, had a second primary cancer, died less than 2 weeks after diagnosis, or had erroneous data entry. Patients could have received neoadjuvant or adjuvant therapy, which were defined as therapy received for a maximum of 90 days before or within 6 months after surgical resection, respectively.
Data Sources
Patient data were accessed via the ICES administrative databases, which were used to collect real-world data through OHIP and other linked population-level provincial or national health databases. Individual patients who met the study inclusion criteria were linked to 10 different databases to retrieve treatment and outcomes data. As described previously [44, 45, 56], these databases provide information on various types of clinical and resource utilization; patient diagnoses, demographics, medication use, and vital statistics were of interest for the current study. Given the nature of administrative databases such as the OCR, patients were not recruited at individual hospitals or office-based clinics; rather, index cancer diagnoses were reported and captured in the OCR and then linked with other administrative databases to determine the study outcomes.
Patient Selection
As the ICES databases do not capture EGFRm status, receipt of EGFR-TKI therapy in the setting of disease recurrence/relapse (i.e., > 6 months after resection) was used as a proxy to presume the presence of an EGFRm. This assumption was made on the basis that EGFR-TKI therapy is the standard of care for patients with sensitizing EGFRm (e.g., exon 19 deletion or exon 21 L858R substitution), which represents the vast majority of EGFRm [15, 52]. Moreover, EGFR-TKI therapies are specifically indicated for these individuals in Canada [15, 37, 53,54,55]. Additionally, as EGFRm occur primarily in non-squamous histology [24, 52], patients with squamous disease were excluded from the overall cohort. Notably, the etiology of non-squamous and squamous disease also differs, providing further rationale for focusing on one histological subtype. The ICES databases are also unable to track disease relapse; therefore, receipt of certain therapies (EGFR-TKIs and platinum doublet chemotherapy) more than 6 months after lung resection was used as a proxy to presume recurrence/relapse of NSCLC.
Using the above criteria, four patient cohorts were defined for analysis: (1) all patients meeting the study inclusion and exclusion criteria; (2) an EGFR-TKI cohort of patients who received an approved EGFR-TKI (gefitinib, erlotinib, afatinib, or osimertinib) via the Ontario Drug Benefit (ODB) plan (which includes patients prescribed oral medication who are either aged ≥ 65 years or < 65 years and on disability or social assistance) > 6 months after resection and were assumed to have experienced disease recurrence/relapse and to have an EGFRm; (3) a non-EGFR-TKI cohort of patients who received platinum doublet chemotherapy > 6 months after resection and were assumed to have experienced disease relapse but were unlikely to have an EGFRm; and (4) a non-relapse cohort of patients who did not receive systemic anti-cancer treatment.
Study Outcomes
All study outcomes were reported at the aggregate level. The proportion of patients who underwent resection for non-squamous stage I–III NSCLC within 180 days of diagnosis in Ontario between April 1, 2010, and March 31, 2019 (the overall cohort), was calculated. In this group, baseline patient characteristics were evaluated (by disease stage at diagnosis where available) and included patient age, sex, geographic location (i.e., whether rural), Charlson comorbidity scores, and the time from diagnosis to resection. Treatment patterns were documented in the overall cohort and by disease stage at diagnosis (where available) and included receipt of neoadjuvant or adjuvant therapy, EGFR-TKIs/chemotherapy for recurrence/relapsed disease, and/or curative or palliative radiation. Overall survival was calculated from the date of resection (as well as the time of therapy initiation for patients with recurrence/relapse) until the end of follow-up or death for each of the four abovementioned cohorts.
Statistical Analysis
Statistical analyses were conducted at ICES and were performed using SAS Enterprise Guide software (version 7.1; SAS Institute, Cary, NC, USA). Patient demographics and baseline characteristics were summarized by number and percentage for categorical variables or by mean and standard deviation (SD) for continuous variables. Survival analyses were stratified to examine the time to loss to follow-up or death from any cause from the date of lung resection or metastasis. Median overall survival (mOS) and 95% confidence intervals (CI) were evaluated using Kaplan–Meier methods for censored data. Survival results were compared in a Kaplan–Meier analysis based on stratification and log-rank test.
Ethical Considerations
The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board (REB #2450–2017) and performed in compliance with Good Clinical Practice (GCP) and Good Pharmacoepidemiology Practice (GPP), including the archiving of essential documents. No patient recruitment or consent was required. Data are retained for 10 years after study completion.
Results
Patient and Clinical Characteristics
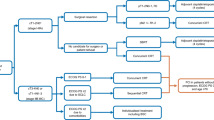
Between April 1, 2010, and March 31, 2019, a total of 83,252 patients were diagnosed with lung cancer in Ontario, Canada (Fig. 1). Of these patients, 21,752 had non-squamous stage I–III NSCLC (stage I: 8721; stage II: 3921; stage III: 9,110). In total, 8255 (38.0%) of these patients (the overall cohort) underwent resection for early-stage lung cancer within 180 days of diagnosis, at rates of 56.0% (n = 4881), 54.2% (n = 2124), and 13.7% (n = 1250) for stage I, II, and III disease, respectively (Table 1). Among those who underwent resection within 180 days of diagnosis, almost two-thirds (63.5%) of patients underwent resection within 60 days of diagnosis. The mean age of patients was 68.0 years (SD 9.1), with slightly more female (53.5%) than male patients (Table 1). The mean Charlson comorbidity scores were 0.61, 0.62, and 0.67 for stage I, II, and III disease, respectively. Approximately 15% of patients lived in a rural setting across all groups.
Treatment
In the overall cohort, adjuvant chemotherapy was received by 1617 patients (19.6%). Use of such therapy was highest among patients with stage III disease (50.0% vs. 3.5% in stage I and 38.7% in stage II; p < 0.001 across disease stages) (Table 1). A total of 195 patients (2.4% of the overall cohort; 19.1% of patients who received EGFR-TKIs/chemotherapy) received an EGFR-TKI via ODB; 825 patients (~ 10%) received chemotherapy in this setting. In the overall cohort, curative radiation was received by 1215 (14.7%) patients and palliative radiation was received by 1276 (15.5%). The proportion of patients receiving these treatments increased with advancing disease.
Overall Survival
In the overall cohort, mOS after the time of resection was 8.2 years (95% CI: 7.8, 8.7) (Fig. 2). When stratified by disease stage at diagnosis and receipt of adjuvant chemotherapy, patients with stage I disease who did not receive adjuvant therapy had prolonged mOS compared with those who did receive such therapy (not estimable [NE] vs. 7.5 years [95% CI: 6.3, NE]) (Fig. 3). Conversely, patients with stage II or III disease who did not receive adjuvant treatment had shorter mOS than those who did receive it (stage II: 4.4 years [95% CI: 4.0, 4.9] vs. 7.6 years [95% CI: 6.5, 8.5]; stage III: 2.7 years [95% CI: 2.3, 3.3] vs. 4.4 years [95% CI: 3.6, 5.1]). The duration of mOS was similar between patients with stage I or stage II disease who received adjuvant chemotherapy and between those with stage II or stage III disease who did not receive adjuvant chemotherapy.
Among patients who received therapy in the recurrence/relapse setting, mOS after resection was approximately 1.5 years longer in the EGFR-TKI cohort than in the non–EGFR-TKI cohort (5.1 years [95% CI: 4.4, 5.8] vs. 3.6 years [95% CI: 3.2, 3.9]) (Fig. 4). Similarly, mOS after disease metastasis was 1.2 years longer in the EGFR-TKI cohort (2.3 years [95% CI: 1.8, 3.0] vs. 1.1 years [95% CI: 1.0, 1.3] in the non-EGFR-TKI cohort (Fig. 5). In the non-relapse cohort of patients who did not receive therapy, mOS was not reached after a median follow-up of 9 years.
Discussion
Numerous treatment options have been developed for locally advanced and metastatic NSCLC over the last 15 years; however, until recently, progress in the management of early-stage disease has remained limited to the introduction of adjuvant cisplatin-based doublet chemotherapy. Although studies of the adjuvant use of early-generation EGFR-TKIs (e.g., gefinitib and erlotinib in the SELECT, RADIANT, ADJUVANT-CTONG, EVAN, and NCIC CTG BR19 trials) have shown some improvements in DFS, no statistically significant benefit in OS was reported. It should be noted that these findings have been challenged because of inappropriate patient selection, poor study design, inadequate treatment duration, and/or limited efficacy [26,27,28,29,30,31,32,33, 57]; still, the early EGFR-TKIs did not receive regulatory approval or the opportunity for reimbursement in Ontario for adjuvant use. In contrast, given recent positive results for osimertinib, atezolizumab, pembrolizumab, durvalumab, and nivolumab in neoadjuvant, adjuvant, and perioperative settings [36, 40,41,42, 58], additional practice-changing therapeutic options are finally becoming available for early-stage NSCLC. Therefore, it is important to understand the real-world characteristics, treatment, and survival of patients who may be eligible for these therapies.
This retrospective analysis represents the most recent evaluation to address this need for real-world data, providing perspective on treatment patterns in Ontario, Canada, and including a study period sufficient to capture survival outcomes across multiple patient subpopulations. Previous Canadian real-world studies have focused on advanced or metastatic NSCLC [44,45,46,47,48] or analyzed considerably older datasets of patients with early-stage resected disease [49,50,51]. In the latter group of studies, patient data were derived from 2001 to 2006, with treatment patterns and survival outcomes examined in relation to patient age (i.e., elderly patients) and timing of adjuvant chemotherapy. These studies also included patients with de novo stage IV disease and were conducted before the availability of certain treatments used in the metastatic setting (e.g., some EGFR-TKIs), which would have impacted their survival results. The current study is therefore unique, having included patients diagnosed with early-stage NSCLC who underwent resection and following them through the long-term progression of their disease. As adjuvant osimertinib is now approved and reimbursed across most of Canada [37], a key goal was to understand the characteristics of patients with an EGFRm.
The current study found that 38% of patients with non-squamous stage I–III NSCLC underwent surgical lung resection, with rates of 56% and 54% observed among those with stage I and II disease, respectively. These relatively low rates are surprising, given that lung resection has been the standard of care for eligible patients with early-stage disease [8, 9]. It is possible, however, that some patients who did not undergo resection received radiotherapy, and specifically stereotactic body radiotherapy, an approach that has increased in use in recent years but was not captured in this study. Regardless, the resection rates for stage I and II disease were generally similar to those reported in an earlier Ontario-based study of stage I—II lung cancer (58.9%; 2010–2012 dataset) [59], as well as those published for other regions [60, 61]. In the previous study of data from Ontario, the authors noted a potential relationship between rates of resection and surgical consultation [59]; however, the latter outcome was not evaluated in the current analysis.
This study also found that in the overall cohort, only 38.7% of stage II and 50.0% of stage III patients received adjuvant chemotherapy after surgical lung resection. In previous real-world investigations of NSCLC conducted in Ontario, utilization rates for adjuvant therapy ranged from 31 [49] (stages I–IV) to 46% [25] (stages IB–IIIA) of patients who underwent resection. The low utilization observed in the current study is concerning, given current guideline recommendations [7,8,9] based on results of the LACE meta-analysis and others: the evidence clearly indicates that adjuvant chemotherapy is associated with a survival benefit among patients with stage II and III NSCLC [10, 62, 63]. Indeed, in the current study, mOS was up to 1.7 times longer among stage II/III patients who had received adjuvant therapy. An alternative, partial explanation for such limited use may relate to patient fitness (e.g., age, frailty), physician preferences, and/or patient concerns related to the potential toxicity of chemotherapy. This finding may also underscore a need for novel adjuvant treatment options during the study period.
Not surprisingly, the overall proportion of patients receiving neoadjuvant chemotherapy was also very low (0.9%) in the current analysis. However, given recent US approval of nivolumab with platinum-doublet chemotherapy (based on CheckMate-816) [64] in this setting, positive interim results for perioperative durvalumab with chemotherapy (AEGEAN trial) [58] and pembrolizumab with chemotherapy (KEYNOTE-671 trial) [65], and ongoing evaluations of other treatments (e.g., osimertinib in NeoADAURA), it will be of interest to monitor the use of neoadjuvant/perioperative therapy and surgery in the near future. Changes in treatment patterns among patients with stage III disease will be of particular interest, should they become eligible for resection after neoadjuvant therapy.
The current study additionally showed that unlike the results for stage II/III disease, patients with stage I disease who received adjuvant chemotherapy had significantly worse mOS than those who went without adjuvant chemotherapy. Although disease substaging details were not available for analysis, this finding is likely explained by the fact that stage IA disease is not typically treated with adjuvant therapy and is associated with better survival outcomes than stage IB [10]. In the overall cohort, 59% of patients had stage I disease, and only 3.5% of these individuals received adjuvant therapy. Additionally, a higher proportion of stage I patients who received adjuvant therapy also received curative or palliative radiation therapy (data not shown), suggesting the presence of larger tumors at baseline. Collectively, these factors may explain the nonintuitive survival results for adjuvant therapy in the stage I population. Regardless, it is recognized that in general, the presence of certain molecular alterations that were not evaluated in this study may have impacted clinical outcomes [66]. For example, a meta-analysis of 41 publications found that patients with KRAS mutations had significantly worse survival than those with wild-type tumors [67].
Another notable observation was that among patients who received therapy in the recurrence/relapse setting, 19.1% (195 of 1020) received an EGFR-TKI via ODB. Although receipt of EGFR-TKI therapy was used as a proxy for EGFRm positivity in this study, this frequency appears to be reasonable, as other studies of EGFRm prevalence have reported frequency of 20–24% in Canadian and North and South American populations [21, 24, 25]. Considering that mOS after resection was longer in the EGFR-TKI cohort than in the non-EGFR-TKI cohort (who received platinum doublet chemotherapy), it becomes evident that patients with EGFRm must be appropriately identified and receive timely therapy with an EGFR-TKI. It should be noted that most EGFR-TKI-treated patients received gefitinib (n = 130), an early-generation therapy that had an mOS of approximately 1.8 years in the phase II IPASS trial (advanced NSCLC EGFRm population) [68]. Although the current real-world study’s results are generally aligned with this finding, increased use of the third-generation EGFR-TKI osimertinib may further extend the survival benefit (mOS in the FLAURA trial was ~ 3.2 years) [69]. Additionally, in the non-relapse cohort, mOS was not reached over 9 years of follow-up. This finding indicates that early-stage patients who do not experience disease relapse may survive to an age similar to that in the general population. Thus, cure is a reasonable expectation and should be the goal for all early-stage patients with resected NSCLC.
This study has both strengths and limitations. The study included a large number of patients, which may permit generalizability of some results to the broader North American population. Most of the study’s limitations are common to retrospective analyses of secondary data. Firstly, the ICES database did not provide information on NSCLC disease substaging (e.g., IA, IIB) at diagnosis, disease stage at treatment initiation, or any patient performance status (e.g., Eastern Cooperative Oncology Group status). These factors can impact treatment selection and subsequent clinical outcomes, including survival. Secondly, as the ICES databases did not capture information on the EGFRm status of patients, EGFR-TKI prescribing in the setting of disease recurrence/relapse was used as a proxy for the presence of the mutation. Therefore, it is possible that some patients with EGFRm were included in the non-EGFR-TKI and non-relapse cohorts. Thirdly, patient status in terms of disease progression was based on the prescribing of medications used to treat disease recurrence/relapse rather than other health record information. As a result, some patients who experienced disease relapse but did not receive subsequent therapy were likely included in the non-relapse cohort. Although prolonged OS (potentially reflecting cure) was observed in this group, the presumably reduced survival among untreated progressed patients likely reduced the estimate. Furthermore, as mentioned previously, the survival results may have been impacted by the presence of unidentified genetic mutations. Fourthly, in Ontario, public funding of oral cancer agents is limited to individuals aged ≥ 65 years or who are on social assistance (regardless of age); accordingly, information for the utilization of these agents among patients aged < 65 years is limited, and given this, analyses were conducted using data from ODB. Finally, there was a need for patient censoring in some cases (e.g., due to loss to follow-up, loss of OHIP coverage), data were potentially missing for some outcomes, and there was a paucity of data on the cause of death.
Conclusions
The results of this study show that among patients with resected, early-stage, non-squamous NSCLC living in Ontario, Canada, adjuvant chemotherapy was received by a relatively small proportion of patients between 2010 and 2019 despite guideline recommendations. Patients who received treatment in the recurrence/relapse setting generally had poor survival, although outcomes varied on the basis of treatment type (and thus by molecular cancer type). Among patients who were assumed to not have experienced relapse, mOS was not reached over 9 years of follow-up. Overall, these findings suggest that the adjuvant treatment options available at the time of the study were underused, and that although cure is possible after resection of NSCLC, many patients still experience disease progression. Accordingly, emerging neoadjuvant and adjuvant treatment options are greatly needed to prevent disease recurrence and improve long-term survival. The study results further indicate that given the fast-moving clinical landscape and evolving options, timely identification of driver mutations will be important for patients with resectable disease as well as those with recurrent/relapsed NSCLC to determine treatment decisions. Once more widely available, the use of liquid biopsy to detect disease recurrence is therefore likely to play a key role in this setting [70]. Collectively, the study findings improve our understanding of the characteristics, treatment, prognosis, and unmet needs of patients with resected, early-stage, non-squamous NSCLC, in turn providing context to the results of recent and ongoing adjuvant clinical studies. As such, the results may be helpful to clinicians and health technology assessment committees making decisions for this population.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to strict enforcement of ICES DAS Policies and Procedures on Privacy and Confidentiality.
Change history
16 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s40487-024-00276-5
References
Canadian Cancer Society. Lung and bronchus cancer statistics. November 2023. Available at: https://cancer.ca/en/cancer-information/cancer-types/lung/statistics. Accessed: 20 Feb 2024. 2023.
Cancer Care Ontario. Ontario Cancer Statistics 2022. Ch 1: Estimated Current Cancer Incidence. Available at: https://www.cancercareontario.ca/en/data-research/view-data/statistical-reports/ontario-cancer-statistics-2022/ch-1-estimated-current-cancer-incidence-2022#:~:text=%5B11%2C12%5D-,Incidence%20by%20Cancer%20Type,11.1%25)%20(Table%201.1). Accessed: 20 Feb 2024. 2022.
Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics: A 2020 special report on lung cancer. Available at: www.cancer.ca/Canadian-Cancer-Statistics-2020-EN. Accessed: 20 Feb 2024. 2020.
Linehan V, Harris S, Bhatia R. An audit of opportunistic lung cancer screening in a Canadian province. J Prim Care Commun Health. 2021;12:21501327211051484.
Darling GE, Tammemägi MC, Schmidt H, et al. Organized lung cancer screening pilot: informing a province-wide program in Ontario. Canada Ann Thorac Surg. 2021;111(6):1805–11.
Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523–31.
Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35(25):2960–74.
Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(4):iv1–21.
Alberta Health Services. Cancer Guidelines. Guideline Resource Unit (GURU), Information for Health Professionals. Lung Treatment Guidelines LU-001, LU-002, LU-003. Non-small Cell Lung Cancer Stages I, II, and III. Last updated: 2012–2014. Available at: https://www.albertahealthservices.ca/info/cancerguidelines.aspx. Accessed: 20 Aug 2021. 2021.
Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9.
Plunkett TA, Chrystal KF, Harper PG. Quality of life and the treatment of advanced lung cancer. Clin Lung Cancer. 2003;5(1):28–32.
Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. Treatment of non-small cell lung cancer (NSCLC). J Thorac D. 2013;5(Suppl 4):S389–96.
Mieras A, Pasman HRW, Klop HT, et al. What goals do patients and oncologists have when starting medical treatment for metastatic lung cancer? Clin Lung Cancer. 2021;22(3):242-51.e5.
Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline update. J Clin Oncol. 2020;38(14):1608–32.
Hanna NH, Robinson AG, Temin S, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) Joint Guideline update. J Clin Oncol. 2021;39(9):1040–91.
Šutić M, Vukić A, Baranašić J, et al. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management. J Pers Med. 2021;11(11):1102.
Villalobos P, Wistuba II. Lung cancer biomarkers. Hematol Oncol Clin North Am. 2017;31(1):13–29.
Mariano C, Bosdet I, Karsan A, et al. A population-based review of the feasibility of platinum-based combination chemotherapy after tyrosine kinase inhibition in EGFR mutation positive non-small cell lung cancer patients with advanced disease. Lung Cancer. 2014;83(1):73–7.
Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892–911.
Ellis PM, Verma S, Sehdev S, Younus J, Leighl NB. Challenges to implementation of an epidermal growth factor receptor testing strategy for non-small-cell lung cancer in a publicly funded health care system. J Thorac Oncol. 2013;8(9):1136–41.
Shiau CJ, Babwah JP, da Cunha SG, et al. Sample features associated with success rates in population-based EGFR mutation testing. J Thorac Oncol. 2014;9(7):947–56.
Graham RP, Treece AL, Lindeman NI, et al. Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med. 2018;142(2):163–7.
Melosky B, Banerji S, Blais N, et al. Canadian consensus: a new systemic treatment algorithm for advanced EGFR-mutated non-small-cell lung cancer. Curr Oncol. 2020;27(2):e146–55.
Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–93.
Kuruvilla MS, Liu G, Syed I, et al. EGFR mutation prevalence, real-world treatment patterns, and outcomes among patients with resected, early-stage, non-small cell lung cancer in Canada. Lung Cancer. 2022;173:58–66.
Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139–48.
Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for Stage II-IIIA (N1–N2) EGFR-mutant NSCLC: final overall survival analysis of CTONG1104 Phase III trial. J Clin Oncol. 2021;39(7):713–22.
Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind. Phase III trial J Clin Oncol. 2015;33(34):4007–14.
Xu ST, Xi JJ, Zhong WZ, et al. The unique spatial-temporal treatment failure patterns of adjuvant gefitinib therapy: a post hoc analysis of the ADJUVANT trial (CTONG 1104). J Thorac Oncol. 2019;14(3):503–12.
Pennell NA, Neal JW, Chaft JE, et al. SELECT: A Phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97–104.
Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol. 2013;31(27):3320–6.
Lin C, Hu F, Chu H, et al. The role of EGFR-TKIs as adjuvant therapy in EGFR mutation-positive early-stage NSCLC: a meta-analysis. Thorac Cancer. 2021;12(7):1084–95.
Li N, Ou W, Ye X, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol. 2014;21(6):2091–6.
Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711–23.
AstraZeneca. Press release: Tagrisso demonstrated strong overall survival benefit in the ADAURA Phase III trial for adjuvant treatment of patients with early-stage EGFR-mutated lung cancer. 9 March 2023. Available at: https://www.astrazeneca.com/media-centre/press-releases/2023/tagrisso-demonstrated-strong-overall-survival-benefit-in-the-adaura-phase-iii-trial.html. Accessed: 24 Apr 2023. 2023
Herbst RS, Wu Y-L, John T, et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non–small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41(10):1830–40.
AstraZeneca (2024) Product Monograph. TAGRISSO(R) (osimertinib tablets). Date of Revision: Jan 02, 2024. Available at: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/tagrisso-product-monograph-en.pdf. Accessed: 16 Feb 2024
European Medicines Agency (2023) Summary of product characteristics for TAGRISSO. Last updated: 20 Nov 2023. Available at: https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf. Accessed: 20 Feb 2024
Food and Drug Administration (2024) Highlights of prescribing information for TAGRISSO (osimertinib) tablets. Revised: 03/2024. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/208065s030lbl.pdf. Accessed: 20 Feb 2024
de Scordilli M, Michelotti A, Bertoli E, De Carlo E, Del Conte A, Bearz A. Targeted therapy and immunotherapy in early-stage non-small cell lung cancer: current evidence and ongoing trials. Int J Mol Sci. 2022;23(13):7222.
Catania C, Muthusamy B, Spitaleri G, Del Signore E, Pennell NA. The new era of immune checkpoint inhibition and target therapy in early-stage non-small cell lung cancer. a review of the literature. Clin Lung Cancer. 2022;23(2):108–15.
Chaft JE, Rimner A, Weder W, Azzoli CG, Kris MG, Cascone T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18(9):547–57.
Statistics Canada (2023) Population estimates, quarterly. Table 17–10–009–01. 2023–03–22. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901. Accessed: 04 May 2023
Seung SJ, Hurry M, Walton RN, Evans WK. Retrospective cohort study of unresectable stage III non-small-cell lung cancer in Canada. Curr Oncol. 2020;27(4):e354–60.
Seung SJ, Hurry M, Walton RN, Evans WK. Real-world treatment patterns and survival in stage IV non-small-cell lung cancer in Canada. J Clin Oncol. 2020;27(4): e361.
Gouran-Savadkoohi M, Mesci A, Pond GR, et al. Contemporary real-world radiotherapy outcomes of unresected locally advanced non-small cell lung cancer. J Thorac Dis. 2023;15(2):423–33.
Perdrizet K, Sutradhar R, Li Q, Liu N, Earle CC, Leighl NB. Second and later-line erlotinib use in non-small cell lung cancer: real world outcomes and practice patterns overtime in Canada. J Thorac Dis. 2021;13(9):5419–29.
Kim YJ, Oremus M, Chen HH, McFarlane T, Fearon D, Horton S. Factors affecting treatment selection and overall survival for first-line EGFR-tyrosine kinase inhibitor therapy in non-small-cell lung cancer. J Comp Eff Res. 2021;10(3):193–206.
Booth CM, Shepherd FA, Peng Y, et al. Time to adjuvant chemotherapy and survival in non-small cell lung cancer: a population-based study. Cancer. 2013;119(6):1243–50.
Booth CM, Shepherd FA, Peng Y, et al. Adjuvant chemotherapy for non-small cell lung cancer: practice patterns and outcomes in the general population of Ontario. Canada J Thorac Oncol. 2012;7(3):559–66.
Cuffe S, Booth CM, Peng Y, et al. Adjuvant chemotherapy for non-small-cell lung cancer in the elderly: a population-based study in Ontario. Canada J Clin Oncol. 2012;30(15):1813–21.
Pretelli G, Spagnolo CC, Ciappina G, Santarpia M, Pasello G. Overview on therapeutic options in uncommon EGFR mutant non-small cell lung cancer (NSCLC): new lights for an unmet medical need. Int J Mol Sci. 2023;24(10):8878.
AstraZeneca (2021) Product Monograph. IRESSA(R) (gefitinib tablets). Date of Revision: March 31, 2021. Available at: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/iressa-product-monograph-en.pdf. Accessed: 16 Feb 2024
Boehringer Ingelheim (2019) Product Monograph. GIOTRIF(R) (afatinib tablets). Date of Revision: June 6, 2019. Available at: https://www.boehringer-ingelheim.com/ca/bipdf/giotrif-product-monograph. Accessed: 16 February 2024
Roche. Product Monograph (2021) TARCEVA(R) (erlotinib tablets). Date of Revision: September 23, 2021. Available at: https://assets.roche.com/f/173850/x/46797b50c0/tarceva_pm_e.pdf. Accessed: 16 February 2024
Seung SJ, Hurry M, Hassan S, Walton RN, Evans WK. Cost-of-illness study for non-small-cell lung cancer using real-world data. Curr Oncol. 2019;26(2):102–7.
Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018;6(11):863–73.
American Association for Cancer Research (2023) Durvalumab-based treatment before and after surgery improved outcomes for patients with resectable non-small cell lung cancer. 16 April 2023. Available at: https://www.aacr.org/about-the-aacr/newsroom/news-releases/durvalumab-based-treatment-before-and-after-surgery-improved-outcomes-for-patients-with-resectable-non-small-cell-lung-cancer/. Accessed: 11 May 2023
Bendzsak AM, Waddell TK, Urbach DR, Darling GE. Surgery and surgical consult rates for early stage lung cancer in Ontario: a population-based study. Ann Thorac Surg. 2017;103(3):906–10.
Khakwani A, Rich AL, Powell HA, et al. Lung cancer survival in England: trends in non-small-cell lung cancer survival over the duration of the National Lung Cancer Audit. Br J Cancer. 2013;109(8):2058–65.
Zeliadt SB, Sekaran NK, Hu EY, et al. Comparison of demographic characteristics, surgical resection patterns, and survival outcomes for veterans and nonveterans with non-small cell lung cancer in the Pacific Northwest. J Thorac Oncol. 2011;6(10):1726–32.
Bradbury P, Sivajohanathan D, Chan A, Kulkarni S, Ung Y, Ellis PM. Postoperative adjuvant systemic therapy in completely resected non-small-cell lung cancer: a systematic review. Clin Lung Cancer. 2017;18(3):259–73.
Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev. 2015;3:011430.
Food and Drug Administration (2023) Highlights of Prescribing Information for OPDIVO (nivolumab). Revised: 2/2023. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125554s119lbl.pdf. Accessed: 19 March 2023
Liberman M, Kato T, Tsuboi M, et al. KEYNOTE-671: Randomized, double-blind, phase 3 study of pembrolizumab or placebo plus platinum-based chemotherapy followed by resection and pembrolizumab or placebo for early stage NSCLC. J Clin Oncol. 2023;41(17):LBA100-LBA.
Aramini B, Banchelli F, Bettelli S, et al. Overall survival in patients with lung adenocarcinoma harboring “niche” mutations: an observational study. Oncotarget. 2020;11(5):550–9.
Pan W, Yang Y, Zhu H, Zhang Y, Zhou R, Sun X. KRAS mutation is a weak, but valid predictor for poor prognosis and treatment outcomes in NSCLC: a meta-analysis of 41 studies. Oncotarget. 2016;7(7):8373–88.
Fukuoka M, Wu Y-L, Thongprasert S, et al. Biomarker analyses and final overall survival results from a Phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74.
Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Souza VGP, Forder A, Brockley LJ, et al. Liquid biopsy in lung cancer: biomarkers for the management of recurrence and metastasis. Int J Molec Sci. 2023;24(10):8894.
Acknowledgements
ICES DAS Policies and Procedures on Privacy and Confidentiality of data were strictly enforced. Once the quality of the data was validated, the ICES Data Covenantor de-identified all personal health information, replacing it with an ICES IKN and transferring it to a “moated” server, making it identifiable for a minimal amount of time. As part of our Data Statement, patient-level data are not available, as all analyses were conducted by ICES DAS in-house and only results were made available to the authors. The authors wish to thank the patients whose data made this study possible.
Medical Writing and Editorial Assistance:
The authors wish to thank Erind Dvorani (ICES DAS Private Sector) for analytical support, as well as Dana L. Anger (WRITRIX Medical Communications Inc.) for medical writing services, which were funded by AstraZeneca Canada Inc. in accordance with Good Publication Practice (GPP) guidelines (http://www.ismpp.org/gpp-2022).
Funding
This work and its publication, including the journal’s Rapid Service Fee, were funded by AstraZeneca Canada Inc.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the review and editing of the manuscript. Study conceptualization was performed by Soo Jin Seung and Geoffrey Liu. Methodology was conducted by Soo Jin Seung, Daniel Moldaver, Shazia Hassan, Iqra Syed, and Geoffrey Liu. Project administration and writing of the original draft were performed by Soo Jin Seung, Daniel Moldaver, Shazia Hassan, and Iqra Syed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
This research was conducted with support from AstraZeneca Canada Inc. The study used de-identified data from the ICES Data Repository, which is managed by ICES with support from its funders and partners: Canada’s Strategy for Patient Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research, and the Government of Ontario. The opinions, results, and conclusions reported herein are those of the authors. No endorsement by ICES or any of its funders or partners is intended or should be inferred. Soo Jin Seung and Shazia Hassan received an unrestricted grant from AstraZeneca Canada Inc. to conduct the study and previously received funding from the Canadian Institute of Health Research, the Ontario Institute for Cancer Research, the Ontario Research Fund, Boehringer Ingelheim Canada, Eisai Limited, and Roche Canada. Shazia Hassan is currently an employee of Cencora. At the time of study conduct, Daniel Moldaver, Iqra Syed, and Mary-Kate Shanahan were employees and shareholders of AstraZeneca Canada Inc. Daniel Moldaver is currently an employee of GlaxoSmithKline. Geoffrey Liu has received institutional research grants from NCI (US), CIHR (Canada), CCSRI (Canada), Amgen, AstraZeneca, Boehringer Ingelheim, and Takeda. He has also received payment or honoraria from AstraZeneca, EMD Serono, Jazz Pharmaceuticals, Novartis, Pfizer, and Takeda, and has participated in data safety monitoring boards or advisory boards with AbbVie, AstraZeneca, BMS, EMD Serono, Jazz Pharmaceuticals, Eli Lilly, Merck, Novartis, Pfizer, Roche, and Takeda.
Ethical Approval
The study was approved by the Sunnybrook Health Sciences Centre Research Ethics Board (REB #2450–2017) and performed in compliance with Good Clinical Practice (GCP) and Good Pharmacoepidemiology Practice (GPP), including the archiving of essential documents. No patient recruitment or consent was required. Data are retained for 10 years after study completion.
Additional information
The original online version of this article was revised: In the Discussion section of this article is discrepancy in a numerical value. In paragraph 4 it reads 28.7% referring to stage II patients. But it should read 38.7%, as per Table 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Seung, S.J., Moldaver, D., Hassan, S. et al. Real-World Treatment Patterns and Survival Among Patients with Stage I–III, Non-Squamous, Non-Small Cell Lung Cancer Receiving Surgery as Primary Treatment. Oncol Ther 12, 311–326 (2024). https://doi.org/10.1007/s40487-024-00268-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-024-00268-5