Abstract
Thrombocytopenia has been reported more frequently with obinutuzumab in clinical trials where it was directly compared with rituximab. However, more significant than the frequency, a unique form of severe thrombocytopenia manifesting very early after the first obinutuzumab infusion has been published in case reports. To further explore this subject, we went through the records of our clinic to identify similar cases of obinutuzumab-induced acute thrombocytopenia (OIAT). Among 24 patients who received obinutuzumab outside of clinical trials, we recovered three cases with OIAT. This paper describes these three cases in detail, placing emphasis on the timing, severity, and the clinical course. Notably, all three patients developed severe OIAT within 5 days of their first obinutuzumab exposure, responded well to transfusion, and recovered within a few days without severe bleeding. None of the patients experienced a similar event in the second course of the obinutuzumab-based therapy. Our observations suggest that OIAT may be a frequent, possibly non-relapsing, and unique event that deserves more attention than it currently receives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We report three cases of obinutuzumab-induced acute thrombocytopenia (OIAT). |
All three cases had refractory lymphoma and developed grade 4 thrombocytopenia within a few days of the first obinutuzumab administration. |
The cases were managed with transfusion and no severe bleeding complicated the course. |
OIAT resolved within a few days and did not recur after the second course of therapy. |
Introduction
The rapid decrease of platelet counts following obinutuzumab infusions was first recognized in a case report [1]. Obinutuzumab-induced acute thrombocytopenia (OIAT) has drawn attention in the last few years; however, the available data remain short of elucidating the key features of what may be a regularly encountered phenomenon. Clinical trials that investigated a large number of patients receiving obinutuzumab (GALLIUM, GAIA-CLL13) reported higher incidences of severe thrombocytopenia in the obinutuzumab arms compared to the rituximab arms; however, the papers did not emphasize the abrupt onset of OIAT [2, 3]. A recent literature review indicated that only five cases of OIAT from real-life settings have been reported to date [4], and this paper was followed by an additional report of four more cases [5]. Considering that OIAT is receiving attention and that there is clearly room for improvement in understanding the setting in which OIAT develops and its natural course, we went through the records of our center to identify similar events and expand the available data.
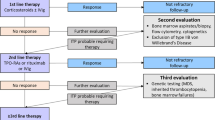
Outside of clinical trials, 24 patients received obinutuzumab between April 2020 and May 2023 in our center. We identified three patients who developed thrombocytopenia following obinutuzumab infusion that could not be explained by the myelosuppressive toxicities of their concurrent medication in terms of timing and the absence of cytopenia in other cell lineages (Fig. 1).
All patients whose data were used in the study were informed of and signed a consent for the use of their medical data for scientific purposes with the following condition that their identities (name, social security number, passport/identity information) remained anonymous. Instructional review board approval was not necessary, and this study was conducted in accordance with the Declaration of Helsinki.
Case Presentations
Patient #1 was an 81-year-old woman with a bulky intra-abdominal mass who was diagnosed with diffuse large B cell lymphoma and failed initial therapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone). Obinutuzumab and lenalidomide were planned for salvage therapy as she was unfit for further cytotoxic chemotherapy. The patient did not have splenomegaly or bone marrow involvement. The two drugs were started concomitantly. The platelet count on the first day (day 1) of therapy was 144 × 109/L. Obinutuzumab infusions (100 mg on day 1, 900 mg on day 2) were uneventful; however, the platelets decreased to 33 × 109/L 36 h after the initial infusion. Pseudothrombocytopenia was ruled out, and as a result of a rapid decline of platelet levels and the patient’s concurrent use of antiplatelet drug (aspirin), 1 unit of pooled platelets was transfused. Prothrombin time (PT), partial thromboplastin time (PTT), and D-dimer were within the normal range, whereas fibrinogen was markedly elevated along with other acute-phase reactants. The platelet count remained stable for the next 2 days and recovered to above 90 × 109/L by day 6, and remained so thereafter. The patient demonstrated only partial response to this treatment and succumbed to progressive disease after four courses of therapy without proceeding to a third line of therapy. The severe thrombocytopenia that developed in the first obinutuzumab administration was not observed in the subsequent infusions.
Patient #2 was a 47-year-old man with grade 2 stage 4 follicular lymphoma (FL) and high tumor burden. He had relapsed 16 months after the completion of R-CHOP while undergoing bimonthly rituximab maintenance therapy. Obinutuzumab-ICE (ifosfamide + carboplatin + etoposide) regimen was initiated for salvage therapy and all four drugs were initiated on day 1 when the platelet count was 111 × 109/L due to moderate splenomegaly and bone marrow involvement by lymphoma. The 900 mg obinutuzumab infusion on day 2 was complicated by a grade 3 infusion reaction (fever, chills, rash, and dyspnea); however, the entire dose was eventually administered. The platelet count decreased to 43 × 109/L and 23 × 109/L on days 3 and 4, respectively. PT, PTT, and fibrinogen levels remained normal, and D-dimer was moderately elevated for a few days. One unit of apheresis platelets was transfused on day 4 because of rapidly declining counts and mucosal bleeding. The platelet levels increased following the transfusion and remained over 50 × 109/L till day 11 of therapy when the level decreased again as expected because of the myelosuppressive toxicity of ICE therapy. The patient went on to receive two subsequent courses of O-ICE followed by high-dose chemotherapy with autologous stem cell (ASC) rescue. The patient remains free of progression into his fourth year after the first O-ICE therapy. He received eight further doses of obinutuzumab maintenance after autologous transplantation without the recurrence of acute thrombocytopenia.
Patient #3 was a 41-year-old man with grade 1 FL who suffered from two bulky intra-abdominal masses and moderate kidney failure due to ureteral obstruction. He had failed R-CHOP therapy in the first year and was initiated on O-ICE therapy on an outpatient basis. The infusion of drugs was uneventful. He presented for a check on day 5 of therapy when a rapid decrease of platelet counts from 112 × 109/L on day 1 to 13 × 109/L was noted. PT, PTT, fibrinogen, and D-dimer levels were within the normal range. Platelet counts between days 2 and 4 were unavailable. The patient demonstrated mild bleeding symptoms and coagulation parameters were unremarkable. He was admitted for transfusion support. The platelet levels appropriately increased after each transfusion; however, he remained transfusion dependent till day 23 of therapy probably because of the toxicity of ICE in the following weeks. The patient went on to receive one more O-ICE, high-dose chemotherapy with ASC rescue and obinutuzumab maintenance. Acute thrombocytopenia was not observed on subsequent obinutuzumab courses. However, during the second year of maintenance, he showed disease progression and has recently been started on rituximab lenalidomide therapy.
Discussion
The initial administration of obinutuzumab seems to be associated with the development of severe thrombocytopenia that develops within a few days of drug exposure in a minority of patients [1, 4,5,6,7]. Similar to most cases described in the literature, OIAT developed in the first administration of obinutuzumab in the three cases we experienced [1, 4, 6]. We did not observe OIAT after repeat administrations of obinutuzumab; however, in most other cases in the literature, anti-CD20 was discontinued or switched to rituximab [4, 6] or thrombocytopenia recurred after subsequent obinutuzumab administrations [5]. In addition to thrombocytopenia, Walter et al. [7] reported the possible association of OIAT with coagulopathy; however, other observations with OIAT [1,2,3,4,5, 8] including our own experience did not share the similar finding.
OIAT is not emphasized in papers reporting data from clinical trials. In the phase 2 GAGE trial studying the single-agent efficacy and safety of obinutuzumab, ten of 78 patients developed grade 3–4 thrombocytopenia; however, none of these events were reported to be acute in onset and no patient discontinued the treatment because of thrombocytopenia in the initial 3 weeks of treatment [9]. The GADOLIN trial, which enrolled 392 patients, compared obinutuzumab and bendamustine combination to single-agent bendamustine. This trial reported a lower incidence of thrombocytopenia in the combination arm and did not report OIAT [10]. The GALLIUM study compared the efficacy and safety of obinutuzumab + chemotherapy to rituximab + chemotherapy in over 1200 patients. Grade 3 or 4 thrombocytopenia was more frequent (6.1% vs. 2.7%) in the obinutuzumab group, but the onset of thrombocytopenia was not discussed [2, 11]. In the recently published GAIA-CLL13 trial, grade 4 thrombocytopenia was more frequent when venetoclax was combined with obinutuzumab (3.9%) than when it was combined with rituximab (1.7%); however, no emphasis was placed on the acute onset [3]. A meta-analysis exploring the adverse effects of obinutuzumab from five different clinical trials concluded that obinutuzumab was associated with a significantly higher rate of thrombocytopenia than rituximab (RR 2.8, 95% CI1.9–4.1) and this event mostly developed in the first course of treatment in patients with higher tumor burden and higher CD20 expression on tumor cells, splenomegaly, and bone marrow infiltration [12]. We consider the observations we reported in the current paper to be among the earliest insights into a particularly severe and early onset thrombocytopenia related to obinutuzumab, strengthening the claim that this adverse event tends to occur with the first dose and suggesting for the first time that repeat administrations may be safe from its recurrence.
Conclusion
The exact frequency of OIAT, the risk factors for its development, and its potential to cause clinically significant bleeding require further exploration. The data from clinical trials confirm that obinutuzumab exposure is associated with thrombocytopenia; however, the acute timing of thrombocytopenia may be underexplored or underemphasized in papers reporting these trials. Our experience in three patients was most significant for the observation that OIAT did not recur with subsequent obinutuzumab administrations, which may be a novel claim about its possibly non-recurring nature. Increased awareness of physicians and researchers for the possibility of OIAT in both clinical trials and real-life settings along with careful reporting of this event by paying particular attention to its timing may allow us to better characterize its features.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sakai K, Matsumura T, Hamada R, Tominaga T, Takahashi T. [Acute thrombocytopenia after obinutuzumab administration in a patient with relapsed follicular lymphoma]. Rinsho Ketsueki. 2020;61(11):1616–9. https://doi.org/10.11406/rinketsu.61.1616.
Marcus R, Davies A, Ando K, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–44. https://doi.org/10.1056/NEJMoa1614598.
Eichhorst B, Niemann CU, Kater AP, et al. First-line venetoclax combinations in chronic lymphocytic leukemia. N Engl J Med. 2023;388(19):1739–54. https://doi.org/10.1056/NEJMoa2213093.
Mechelfekh Y, Pontrucher A, Paillassa J, Temple M, Houot R. Obinutuzumab-induced acute thrombocytopenia: report of two cases and review of literature. Br J Haematol. 2023. https://doi.org/10.1111/bjh.18826.
Ng JY, Joshi M, Choi P. Frequency and outcomes of obinutuzumab-induced thrombocytopenia. Br J Haematol. 2023. https://doi.org/10.1111/bjh.19147.
Haage TR, Surov A, Mougiakakos D, Berisha M. Successful use of intravenous immunoglobulins in an obinutuzumab-related acute thrombocytopenia. Hema. 2022;6(8):e751.
Walter HS, Jayne S, Mensah P, Miall FM, Lyttelton M, Dyer MJ. Obinutuzumab-induced coagulopathy in chronic lymphocytic leukaemia with trisomy 12. Blood Cancer J. 2016;6(6):e435. https://doi.org/10.1038/bcj.2016.42.
Aslam HA, Martinez CSV, Jayananda S, Liles D, Weil A. Obinutuzumab induced ITP: a case report. Blood. 2022;140(Suppl 1):11926–7. https://doi.org/10.1182/blood-2022-171039.
Byrd JC, Flynn JM, Kipps TJ, et al. Randomized phase 2 study of obinutuzumab monotherapy in symptomatic, previously untreated chronic lymphocytic leukemia. Blood. 2016;127(1):79–86. https://doi.org/10.1182/blood-2015-03-634394.
Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–93. https://doi.org/10.1016/S1470-2045(16)30097-3.
Hiddemann W, Barbui AM, Canales MA, et al. Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J Clin Oncol. 2018;36(23):2395–404. https://doi.org/10.1200/JCO.2017.76.8960.
Amitai I, Gafter-Gvili A, Shargian-Alon L, Raanani P, Gurion R. Obinutuzumab-related adverse events: a systematic review and meta-analysis. Hematol Oncol. 2021;39(2):215–21. https://doi.org/10.1002/hon.2828.
Funding
No funding was received for this study or publication of this article.
Author information
Authors and Affiliations
Contributions
Umut Yılmaz collected the study data and prepared the manuscript. Selin Küçükyurt was involved in data collection and medical care of included subjects. Muhlis Cem Ar was involved in medical care of included subjects. Ahmet Emre Eşkazan was responsible for the concept and design as well as critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Umut Yılmaz, Selin Küçükyurt, Muhlis Cem Ar, and Ahmet Emre Eşkazan have nothing to disclose.
Ethical Approval
All patients whose data were used in the study were informed of and signed a consent for the use of their medical data for scientific purposes with the following condition that their identities (name, social security number, passport/identity information) remained anonymous. Instutional review board approval was not necessary, and this study was conducted in accordance with the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yılmaz, U., Küçükyurt, S., Ar, M.C. et al. Acute Thrombocytopenia Complicating the Initial Administration of Obinutuzumab: Is It More Frequent Than We Think?. Oncol Ther 12, 157–161 (2024). https://doi.org/10.1007/s40487-023-00259-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-023-00259-y