Abstract
Introduction
Chronic myeloid leukemia (CML) is a chronic disease with treatment-free remission (TFR) increasingly regarded as a feasible goal of treatment. However, various factors may influence adherence to international guidelines for CML management. This study aimed to compare the reporting of care between patients with CML and their treating doctors.
Methods
Parallel patient and physician online surveys were conducted between September 22, 2021, and March 15, 2022, which focused on the perceptions of 1882 adult patients with CML and 305 physicians regarding tyrosine kinase inhibitor (TKI) treatment options, monitoring and toxicities, TFR, and challenges faced.
Results
Among the enrolled patients, 69.9% received first-line imatinib treatment, 18.6% received nilotinib, and 4.7% received dasatinib. Among the patients treated with imatinib, 36.7% switched to other TKIs due to imatinib resistance/intolerance (71.1%), exploration of more potent TKIs to achieve TFR (8.9%), and treating physicians’ recommendation (14.0%), with a median duration of initial treatment of 14 months [interquartile range (IQR) 6–36]. Most (91.8%) physicians agreed that the breakpoint cluster region–Abelson 1 (BCR::ABL1) transcript level should be assessed every 3 months, but only 42.7% of individuals committed to 3-monthly testing and only 17.8% strictly followed their treating physicians’ recommendation. Half of the patients aimed for TFR; however, just 45.2% of physicians considered TFR as one of the top three goals for their patients. The major concern in obtaining TFR was patients’ adherence. Fatigue was often distressing for patients with TKIs, while physicians were more concerned about platelet and neutrophil counts. A total of 12% and 20.8% of patients reported moderate/severe anxiety and depression, respectively, while only 53.7% of physicians had concerns about their patients’ mental health. During the coronavirus disease 2019 (COVID-19) pandemic, 69.2% of patients reported a reduction in their income. Among these patients, 61.8% maintained their current treatment, while 7.3% switched to cheaper alternatives or discontinued treatment, with over 80% of these patients belonging to the low-income group.
Conclusions
Overcoming challenges in patient–physician communication and treatment access is key to improving disease management and quality of life, especially for patients with low income.
Trial Registration
ClinicalTrials.gov identifier NCT05092048.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Achieving an optimal outcome in chronic myeloid leukemia (CML) requires both prolonged adherence to oral tyrosine kinase inhibitor (TKI) therapy by patients and careful monitoring of treatment responses by their physicians. Real-world evidence has revealed that guideline recommendations for CML management have not been consistently implemented, and considerations for monitoring responses to TKIs have not been sufficiently investigated. |
In this setting, it is necessary to explore the discrepancies between patient and physician reporting on CML treatment and obstacles to adhering to guideline recommendations. |
Overcoming challenges in patient–physician communication and treatment access are key to improving disease management and quality of life, especially for patients with low income. |
Introduction
Achieving an optimal outcome in chronic myeloid leukemia (CML) requires both prolonged adherence to oral tyrosine kinase inhibitor (TKI) therapy by patients and careful monitoring of treatment responses by their physicians [1, 2]. Nevertheless, long-term treatment with TKIs is accompanied by high cost and the risk of adverse events (AEs), which negatively impact patients’ quality of life (QoL) and can potentially cause significant morbidity and mortality [3,4,5,6]. Nowadays, doctors and patients are keen to consider TKI discontinuation in clinical practice [7,8,9]; however, achieving long-term treatment-free remission (TFR) is only possible in 10–20% of patients [10,11,12].
The discrepancies in reporting health status and symptom severity between patients with CML and their treating physicians were previously reported by Efficace et al. [13]; however, limited data exist describing the factors that influence TKI treatment choice, disease monitoring, and treatment objectives among them. Various factors (such as technical, financial, and educational challenges) may prevent the implementation of international guidelines for CML management in the clinical routine [14,15,16]. In an era of patient-centered care [17], given the lifelong therapy needed for most patients with CML [18], a possible mismatch in such reporting (including treatment options, TFR, patients’ emotional and psychological needs, challenges faced by physicians) could have important clinical implications, especially during the COVID-19 pandemic.
In this study, parallel patient and physician questionnaires were developed to collect information on TKI use with the aim of comparing the reporting of care between patients with CML and their treating doctors. Indeed, the availability of comparative evidence-based information from both the patient’s and physician’s standpoint would offer a unique, patient–physician joint perspective that complements current guidance and literature, which would help guide patient and physician decision-making and may improve patient–physician relationships.
Methods
Survey Design and Participants
In China, a steering committee of expert CML clinicians from 20 level A tertiary care centers developed the physician- and patient-based surveys. Eligible hematologist/oncologist participants were those who had completed their medical subspecialty training and were responsible for treating five or more adult patients with chronic-phase CML who had received the first-line TKI therapy. Patient survey invitations were sent via email to physicians, who were responsible for further dissemination to patients during routine consultations following the approval of the study in their center. Inclusion criteria for patient participants included age ≥ 18 years, initial diagnosis of Philadelphia chromosome-positive and/or BCR::ABL1-positive disease in the chronic phase, and receipt of first-line TKI treatment. Patients diagnosed at the acute phase/blast phase or receiving other treatment were not eligible. This self-administrated, cross-sectional, online survey was completed between September 22, 2021, and March 15, 2022 using the WeChat-based survey program, Wenjuanxing.
This comparative test between patients and physician was performed using questionnaires with similar subjects, but the questions asked were tailored to each group. The anonymous questionnaire for patients with CML included four sections: demographics (7 questions), CML symptoms and treatment (25 questions), impact of the COVID-19 pandemic (14 questions), and mental health (16 questions). All enrolled patients provided written informed consent ahead of the survey. The anonymous questionnaire for physicians comprised six sections: demographics (7 questions), CML diagnosis and monitoring (11 questions), CML treatment and efficacy (20 questions), TFR (6 questions), perceptions of patients (9 questions), and attitudes toward current CML guidelines and knowledge sought regarding CML (5 questions). Monthly income was collected in Chinese renminbi (5000 RMB) and reported in USD ($700 USD, rounded to the nearest $50 USD), a sum that amounted to the per capita monthly income of primary and secondary school teachers in China. The complete survey questionnaire and responses are provided in the Supplementary Materials.
The study was reviewed by the Medical Ethics Committee of Southern Medical University Nanfang Hospital, which confirmed that the research qualified as exempt from medical ethical review under Article 1, “Conducting research using legally obtained public data or data generated through observation without interfering with public behavior,” as outlined in the exemption from medical ethics review instructions. Therefore, the application for exemption from medical ethics review for this study was approved. All individual entrants who wished to participate had to provide consent via a tick/checkbox before beginning the survey, and the trial was conducted in accordance with the Declaration of Helsinki. This study was registered at www.clinicaltrials.gov (ClinicalTrials.gov identifier NCT05092048).
Measurements
The two most common mental disorders—depression and anxiety—were assessed in the patient survey. Depression was assessed using the Patient Health Questionnaire–9 scale (PHQ-9) [19], which consists of nine items. Anxiety was assessed by the Generalized Anxiety Disorder–7 scale (GAD-7) [20], which consists of seven terms. These two scales were designed to quantify the degree of anxiety and depressive symptoms and have been widely used for patient self-assessment. Participants were asked how often they had experienced each symptom over the past 2 weeks, with options including “not at all,” “several days,” “more than half the days,” and “nearly every day,” scored as 0, 1, 2, and 3, respectively. A total score equal to or greater than 10 [19, 20] on the PHQ-9 and GAD-7 indicates moderate to severe depression and anxiety, respectively.
Statistical Analyses
Continuous data are reported as median (interquartile range [IQR]) and categorical variables as counts and percentages and their 95% confidence intervals (CIs). Categorical variables were compared with the chi-square test. A value of p < 0.05 was considered statistically significant. Analyses were conducted with IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 305 physicians and 1882 patients from China were enrolled in the survey between September 22, 2021, and March 15, 2022. Most of the patients (57.4%) were male, with median age at diagnosis of 37 years (range: 7–79) and median duration of CML of 48 months (IQR 22–84). Among patients, 619 (32.9%) had more than 12 years of education (e.g., university degree), and 1446 (76.8%) had monthly income ≤ $700 USD. Among the physician respondents, 22.3% had been treating patients with CML for 5–10 years, and 55.4% for > 10 years (Table 1).
TKI Treatment
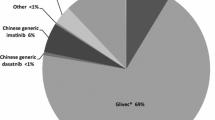
A total of 1316 patients (69.9%, n = 1316/1882) were on imatinib, 350 (18.6%, n = 350/1882) were on nilotinib, 89 (4.7%, n = 89/1882) were on dasatinib, and 127 (6.7%, n = 127/1882) were on other treatment options (such as flumatinib and radotinib) as first-line treatment (Table 2). Significantly, a larger proportion of patients with monthly income > $700 USD were on branded imatinib as initial treatment than those with monthly income ≤ $700 USD (63.7% vs. 47.7%; p < 0.001).
Among the patients with imatinib as first-line treatment, 36.8% (n = 484/1316) had experienced TKI switch, with a median duration of initial imatinib treatment of 14 months (IQR 6–36), and alternative treatment mainly included nilotinib (57.0%, n = 276/484) (Table 2). Reasons for TKI switch were imatinib resistance/intolerance (71.1%, n = 344/484), exploration of more potent TKIs to achieve TFR (8.9%, n = 43/484), treating physicians’ recommendation (14.0%, n = 68/484), and others (6.0%, n = 29/484), such as cost issues or local reimbursement policy. A total of 1442 (76.6%, n = 1442/1882) patient participants reported that they had achieved the treatment milestone (BCR::ABL1IS level of ≤ 10% at 3 months; BCR::ABL1IS level of ≤ 1% at 6 months; BCR::ABL1IS level of ≤ 0.1% at 12 months). Among these patients, 26.9% (n = 389/1442) reported having experienced a switch in TKI treatment. Among the remaining 23.4% (n = 440/1882) of patients who reported failing to achieve the treatment milestone, 45.6% (n = 201/440) had experienced a switch in TKI treatment.
The first-line use of second-generation TKIs was widely considered by the responding physicians (91.1% n = 278/305). However, in clinical practice, 86.9% (n = 265/305) of physicians use imatinib as the first-line treatment. Top factors in determining the first-line treatment were patients’ prognosis and comorbidities (83.6%, n = 255/305), drug price (78.7%, n = 240/305), and patients’ treatment goals (survival/TFR, 45.2%, n = 138/305).
In terms of therapy selection considerations, both physicians and patients rated efficacy (88.9%, n = 271/305 and 77.4%, n = 1457/1882), safety (71.5%, n = 218/305 and 64.7%, n = 1218/1882), and cost (64.6%, n = 197/305 and 72.2%, n = 1358/1882) as the top three considerations. Notably, 48.5% (n = 148/305) of physicians believed there was no difference in efficacy between branded and generic TKIs, while 51.5% (n = 157/305) believed that generics had inferior efficacy, and physicians with more than 10 years of experience in treating tended to believe in comparable efficacy between the two forms (Supplementary Materials Figure S1A). However, most patients did not know (66.4%, n = 1249/1882) whether there was a difference between the two forms, and only 8.9% (n = 168/1882) reported comparable efficacy (Supplementary Materials Figure S1B).
Disease Monitoring
Only 42.7% (n = 803/1882) of the patients reported that they generally monitored BCR::ABL1 levels every 3 months, and only 17.8% (n = 335/1882) strictly followed their treating physicians’ recommendations (Fig. 1). Among the 76.6% (n = 1442/1882) of patients who reported meeting response milestones at 3, 6, and 12 months, lower proportions were currently performing BCR::ABL1 level assessment every 3 months or were strictly adherent to their treating physicians’ recommendations than among those who did not sustainably reach response milestones (57.3% vs. 70.9%, p < 0.001). Although higher monthly income (> $700 USD) was supposed to be a favorable factor for complying with scheduled molecular monitoring, we found no significant difference on this in our study (61.7% vs. 60.1%, p = 0.549) (Supplementary Materials Figure S2).
Physician versus patient respondents reporting disease monitoring frequency: 92.5% of the physicians’ (N = 305) ideal BCR::ABL1 testing frequency was every 3 months; among them, only 83.2% achieved this in clinical practice, while only 42.7% of patient respondents adhered to 3-monthly testing, and only 17.8% strictly followed their treating physicians’ recommendations. BCR::ABL1 breakpoint cluster region–Abelson 1
In contrast, most (92.5%, n = 280/305) physician respondents believed that BCR::ABL1 levels should be assessed every 3 months, while such practice was achieved in 83.2% (n = 233/280) of patients with CML in our study (Fig. 1). The main reasons for suboptimal treatment response monitoring cited by physicians were cost (73.1%, n = 223/305), lack of coordination between testing and patient visits (58.4%, n = 178/305), patients not wanting to know their own condition (48.2%, n = 147/305), and limited laboratory capability such as inaccessibility to a standardized polymerase chain reaction (PCR) test (17.0%, n = 52/305).
Treatment Objectives
Half (49.9%, n = 940/1882) of patient respondents stated that their treatment objective was TFR, and we found that patients with monthly income > $700 USD or patients who were well educated were more likely to have TFR as their goal, than patients with ≤ $700 USD income (60.1% vs. 46.9%, p < 0.001) or with a low level of education (62.3% vs. 43.8%, p < 0.001). It is worth noting that 47.5% (n = 894/1882) of the patients (Table 1) reported that they lacked awareness of TFR, whereas the remainder of the patients (52.5%, n = 988/1882) who reported that they were well informed had a higher proportion who desired to achieve TFR (53.6% vs. 45.9%, p = 0.001).
Overall, physician respondents rated the top three treatment objectives as achievement of deep molecular response (DMR; 77.4%, n = 236/305), prolongation of overall survival (63.3%, n = 193/305), and achievement of early molecular response (EMR; 54.8%, n = 167/305); those priorities were nearly unanimous among physicians of various years of experience (Fig. 2). Nearly all (94.4%, n = 288/305) of the physicians embraced the idea of TFR, and they believed that patients who persistently obtained optimal response to TKI treatment (92.0%, n = 265/288), with a low-risk Sokal score (49.3%, n = 142/288) and who strongly desired to stop TKIs (46.5%, n = 134/288), may be more likely to attempt TFR. Physician-reported major barriers for attempting TFR in current practice were patients’ poor adherence in disease monitoring after medication cessation (61.5%, n = 177/288), refusal for TFR because of worries about recurrence (56.6%, n = 163/288), and lack of patient candidates to discontinue medication (45.5%, n = 131/288).
Treatment expectations in 305 physician respondents by number of years treating CML, among the above six potential treatment objectives. Most physicians considered DMR, OS, and EMR as the top three important treatment objectives. CML chronic myeloid leukemia; AP accelerated phase; BC blast crisis; DMR deep molecular response; EMR early molecular response; TFR treatment-free remission; OS overall survival; QoL quality of life
Regarding the question “If taking a drug makes it possible to discontinue TKI treatment in the future, what is your concern?”, the success rate of discontinuing TKIs (92.8%, n = 283/305), molecular relapse after TKI withdrawal (64.9%, n = 198/305), and medication duration before TKI withdrawal (58.0%, n = 177/305) were three major concerns among physician respondents. The majority of patients (57.4%, n = 1080/1882) were concerned about the success rate of discontinuing TKIs, followed by molecular relapse after TKI withdrawal (23.0%, n = 433/1882), costs before TKI withdrawal (12.4%, n = 234/1882), medication duration before TKI withdrawal (4.9%, n = 93/1882), and molecular monitoring frequency during TKI withdrawal (2.2%, n = 42/1882). Cost before TKI withdrawal was the greatest concern in 12.4% patients, but was of less concern among doctors.
Treatment-Related Toxicities
Of all symptoms listed, patients more often reported certain less life-threatening symptoms as more severe than their physicians. The hematological adverse event (HAE) anemia (42.2%, n = 794/1882) was most commonly reported by patients, while physicians were more likely to report thrombocytopenia (83.6%, n = 255/305) and neutropenia (87.5%, n = 267/305); the non-hematological adverse event (non-HAE) fatigue (31.6%, n = 594/1882) was most reported by patients, whereas physicians were more concerned with edema (69.8%, n = 213/305), pleural effusion (59.3%, n = 181/305), digestive symptoms (58.0%, n = 177/305), and rash and pruritus (54.4%, n = 166/305) (Fig. 3). The mental health of patients was assessed by the PHQ-9 and the GAD-7(Supplementary Materials Figure S1), with 12.0% (n = 225/1882; GAD-7 score ≥ 10, 95% CI: 10.5–13.4%) of patients experiencing anxiety and 20.8% (n = 391/1882; PHQ-9 score ≥ 10, 95% CI: 18.9–22.6%) experiencing depression (Table 3). It was also found that patients who experienced anemia were associated with higher prevalence of anxiety than with other HAEs, and fatigue and abdominal discomfort were associated with higher risk of anxiety and depression compared with other non-HAEs (Table 3).
Percent of AEs reported by patients (N = 1882) and physicians (N = 305) that had the most negative influence on QoL with regard to TKI therapy. A Most patients stated that anemia among the HAEs had the most negative influence on their QoL; B Most patients stated that fatigue among the non-HAEs had the most negative influence on their QoL; C Most physicians noted that thrombocytopenia and neutropenia among the HAEs had the most negative influence on their patients' QoL; D Most physicians noted that rash and pruritus, edema, digestive symptoms, and pleural effusion among the non-HAEs had the most negative influence on their patients' QoL. AEs adverse events; HAEs hematological adverse events; QoL quality of life; TKI tyrosine kinase inhibitor
The Effects of the COVID-19 Pandemic on CML Treatment
Most patient respondents (86.3%, n = 1624/1882) claimed that they performed follow-up visits and molecular monitoring as scheduled. Obstacles to treatment cited by patients were mainly travel restriction (43.9%, n = 827/1882) or worry about the risk of SARS-CoV-2 infection (35.5%, n = 668/1882). In contrast, up to 40.7% (n = 124/305) of physician respondents reported that less than half of their patients performed the requested visits and tests. In comparison with physician-reported obstacles, patient perception of monitoring as unnecessary (55.1%, n = 168/305) and reduced income due to the pandemic (32.5%, n = 99/305) were also critical factors, which may explain the mismatch in patient and physician reporting.
Significantly, 69.2% (n = 1302/1882) of the patients reported that the COVID-19 pandemic had affected their income, consequently impacting the treatment of CML. Among these patients, 61.8% (n = 1164/1882) had maintained their current treatment, while 7.3% (n = 138/1882) had switched to cheaper alternatives or discontinued treatment. Notably, most of the patients (> 80%) reporting being affected by the COVID-19 pandemic were in the low-income group (monthly income ≤ $700 USD).
Discussion
In CML care, treatment decisions increasingly incorporate patient and physician preferences regarding multiple aspects of quality of life, practicality, and cost-effectiveness [21]. Previous studies in CML have found poor to moderate agreement between physician and patient reports of symptom severity, health status, and pain after TKI discontinuation [13, 22]. There is convincing evidence that both physician and patient full adherence to therapy is critical to attaining and maintaining an optimal response [23,24,25]. Our study focused on comparing perceptions regarding treatment choice, treatment response monitoring, TFR, and toxicities between patients with CML and their physicians and sought to understand issues requiring improvement to support shared decision-making and improve the patient–physician relationship.
The selection of first-line treatment in this study was determined primarily by considering its efficacy, safety, and cost from the perspective of both the physician and patient. Imatinib was administered to 69.9% of newly diagnosed patients, aligning with the guideline recommendation of imatinib as the most cost-effective therapy in chronic-phase CML [26, 27]. Among the patients initially treated with imatinib, 36.7% changed treatment, with a median duration of 14 (IQR: 6–36) months. This finding is consistent with a previous European study showing that intolerance was a key driver for switching, and patients with chronic-phase CML who did not switch TKIs were more likely to achieve clinical response [28]. Furthermore, roughly half of the physicians felt that branded imatinib was superior to its generic counterpart. As there is solid evidence of similar efficacy [29], further studies are needed to explore adherence among patients receiving generic and branded TKIs.
It has been demonstrated that patient adherence to guideline recommendations on molecular monitoring contributes to better clinical outcomes [2, 15]. In our study, a significant proportion of patients (76.7%) reported achieving response milestones at 3, 6, and 12 months. However, only 57.3% of patients were currently committed to 3-monthly testing/strictly following their doctors’ recommendations. Fortunately, among the patients (23.3%) who did not reach response milestones, a higher proportion (70.9%) performed BCR::ABL1 level assessment according to the guidelines.
To date, achievement of TFR remains an attractive and desirable goal [30], but it is achieved in only 10–20% of patients in the real world [31]. An interesting finding is that TFR (45.2%) was not considered as one of the top three treatment goals by the majority of physicians; most of them considered DMR (77.4%) a prerequisite for attempting TFR [32,33,34] as the primary goal of therapy for their patients in our study. It should be noted that poor adherence and worrying about recurrence were major hurdles to attempting TFR for CML. The higher success rate of withdrawal of TKIs in real-world scenarios may serve as an incentive for patients to attempt discontinuation. The occurrence of molecular relapse following cessation of TKI treatment may be more significant in terms of disease progression. In our study, both physicians and patients attached significant importance to these two aspects, with a greater emphasis on the success rate of discontinuing TKIs. This suggests that physicians should provide more detailed information about these two questions during routine clinical practice.
Long-term AEs negatively affect patients’ QoL, resulting in decreased adherence to therapy [35]. Fatigue and anemia were often distressing for patients with TKIs, while physicians tended to prioritize monitoring platelet and neutrophil counts. Anxiety and depression have been identified in patients with CML [36]. Although having a seemingly low incidence in our survey, it was significantly higher than in a generally healthy population in a study conducted by Jiang et al. [36] (depression: 20.8% vs. 8.0%, anxiety: 12.0% vs. 5.0%). The most clinically relevant finding is that patients who reported fatigue and anemia which physicians largely ignored were associated with higher incidence of mental disorders. Nevertheless, just 41.3% of patients expressed a desire for increased support from their physician, and 53.7% of physicians acknowledged the importance of addressing their patients’ psychological problems.
For patients with CML, the regularity of clinic visits and frequency of molecular monitoring was significantly reduced during the COVID-19 pandemic, deviating significantly from guidelines [34]. In this study, 69.2% of patients reported that their income had decreased, leading 7.3% of them to switch to cheaper alternatives or to discontinue CML treatment. It should be noted that most of them (> 80%) belonged to the low-income group.
This study has several limitations: Firstly, anxiety and depression were assessed through self-reported questionnaires rather than diagnosis by medical professionals, and they were not concurrently compared with the general population; therefore, we have included a comparison of our data with that by Jiang et al. [36]. Secondly, our study is limited to the use of phone questionnaires, which might introduce bias in our sample, especially in ignoring patients without smartphones. Thirdly, the questions were presented with fixed, multiple-choice, or Likert scale responses (not open-ended questions with free-text answers), and there was no opportunity to explore respondent answers further. Lastly, our results may be limited due to potential variations in treatment reimbursement across different countries.
Conclusions
This prospective exploratory study, based on a large sample of patients recruited in multiple centers, demonstrated that physicians and patients basically align on treatment options, TFR, and treatment challenges, also identifying factors that physicians largely ignored, such as symptoms with the most negative impact. Understanding these gaps between physicians and patients would contribute to treatment optimization and increase the proportion of potential candidates eligible to attempt TFR in the long term.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Keating N, Brooks G, Landrum M, et al. The oncology care model and adherence to oral cancer drugs: a difference-in-differences analysis. J Natl Cancer Inst. 2022;114(6):871–7.
White HE, Salmon M, Albano F, et al. Standardization of molecular monitoring of CML: results and recommendations from the European treatment and outcome study. Leukemia. 2022;36(7):1834–42.
Atallah E, Schiffer CA, Radich JP, et al. Assessment of outcomes after stopping tyrosine kinase inhibitors among patients with chronic myeloid leukemia: a nonrandomized clinical trial. JAMA Oncol. 2021;7(1):42–50.
Inzoli E, Aroldi A, Piazza R, Gambacorti-Passerini C. Tyrosine kinase inhibitor discontinuation in chronic myeloid leukemia: eligibility criteria and predictors of success. Am J Hematol. 2022;97(8):1075–85.
Janssen L, Blijlevens N, Drissen M, et al. Fatigue in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapy: predictors and the relationship with physical activity. Haematologica. 2021;106(7):1876–82.
Breccia M, Chiodi F, Nardozza A, et al. The economic burden of chronic myeloid leukemia in patients with later lines: findings from a real-world analysis in Italy. Adv Ther. 2023;40(3):961–74.
Fava C, Rege-Cambrin G, Dogliotti I, et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104(8):1589–96.
Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94(3):346–57.
Ono T. Which tyrosine kinase inhibitors should be selected as the first-line treatment for chronic myelogenous leukemia in chronic phase? Cancers (Basel). 2021;13(20):5116.
Etienne G, Dulucq S, Bauduer F, et al. Incidences of deep molecular responses and treatment-free remission in de novo CP-CML patients. Cancers (Basel). 2020;12(9):2521.
Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–57.
Rousselot P, Loiseau C, Delord M, Cayuela J, Spentchian M. Late molecular recurrences in patients with chronic myeloid leukemia experiencing treatment-free remission. Blood Adv. 2020;4(13):3034–40.
Efficace F, Rosti G, Aaronson N, et al. Patient- versus physician-reporting of symptoms and health status in chronic myeloid leukemia. Haematologica. 2014;99(4):788–93.
Turkina A, Wang J, Mathews V, et al. TARGET: a survey of real-world management of chronic myeloid leukaemia across 33 countries. Br J Haematol. 2020;190(6):869–76.
Goldberg SL, Akard LP, Dugan MJ, Faderl S, Pecora AL. Barriers to physician adherence to evidence-based monitoring guidelines in chronic myelogenous leukemia. J Oncol Pract. 2015;11(3):e398-404.
Yu L, Wang H, Gale R, et al. Impact of socio-demographic co-variates on prognosis, tyrosine kinase-inhibitor use and outcomes in persons with newly-diagnosed chronic myeloid leukaemia. J Cancer Res Clin Oncol. 2022;148(2):449–59.
Gilligan T, Salmi L, Enzinger A. Patient–clinician communication is a joint creation: working together toward well-being. Am Soc Clin Oncol Educ Book. 2018;38:532–529.
Sharf G, Marin C, Bradley J, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34(8):2102–12.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Ector GICG, Govers TM, Westerweel PE, Grutters JPC, Blijlevens NMA. The potential health gain and cost savings of improving adherence in chronic myeloid leukemia. Leuk Lymphoma. 2019;60(6):1485–92.
Flynn KE, Atallah E, Lin L, et al. Patient- and physician-reported pain after tyrosine kinase inhibitor discontinuation among patients with chronic myeloid leukemia. Haematologica. 2022;107(11):2641–9.
Efficace F, Cottone F, Yanez B, et al. Patient-reported symptom monitoring and adherence to therapy in patients with newly diagnosed chronic myeloid leukemia. Cancer. 2023. https://doi.org/10.1002/cncr.35021.
Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–8.
Obeng-Kusi M, MacDonald K, van Lierde MA, Lee CS, De Geest S, Abraham I. No margin for non-adherence: probabilistic Kaplan–Meier modeling of imatinib non-adherence and treatment response in CML (ADAGIO study). Leuk Res. 2021;111: 106734.
Yamamoto C, Nakashima H, Ikeda T, et al. Analysis of the cost-effectiveness of treatment strategies for CML with incorporation of treatment discontinuation. Blood Adv. 2019;3(21):3266–77.
Hochhaus A, Baccarani M, Silver R, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84.
Gambacorti-Passerini C, Chen C, Davis C, et al. Treatment patterns and clinical outcomes of tyrosine kinase inhibitors in chronic-phase CML in clinical practice: 3-year European SIMPLICITY data. Eur J Haematol. 2021;106(1):82–9.
Abou Dalle I, Kantarjian H, Burger J, et al. Efficacy and safety of generic imatinib after switching from original imatinib in patients treated for chronic myeloid leukemia in the United States. Cancer Med. 2019;8(15):6559–65.
Saifullah HH, Lucas CM. Treatment-free remission in chronic myeloid leukemia: can we identify prognostic factors? Cancers (Basel). 2021;13(16):4175.
Garcia-Horton A, Lipton JH. Treatment outcomes in chronic myeloid leukemia: does one size fit all? J Natl Compr Canc Netw. 2020;18(10):1421–8.
Gugliotta G, Castagnetti F, Breccia M, et al. Treatment-free remission in chronic myeloid leukemia patients treated front-line with nilotinib: 10-year follow-up of the GIMEMA CML 0307 study. Haematologica. 2022;107(10):2356–64.
Hehlmann R. The new ELN recommendations for treating CML. J Clin Med. 2020;9(11):3671.
Deininger M, Shah N, Altman J, et al. Chronic myeloid leukemia, version 2.2021 NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(10):1385–415.
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691–709.
Bao M, Yang S, Gale RP, et al. Mental health in persons with chronic myeloid leukemia during the SARS-CoV-2 pandemic: the need for increased access to health care services. Front Psychiatry. 2021;12: 679932.
Acknowledgements
We thank the patients and physicians for consenting to participate.
Funding
This work was supported by the Open Project of Yunnan Blood Disease Clinical Medical Center (2020LCZXKF-XY05); the Key Basic Research Project of Guangzhou City-Basic and the Applied Basic Research Program of Science (202201011781); National Natural Science Foundation of China (82160039, 81700162); Natural Science Foundation of Guangdong Province (2020A1515010409); and Guangzhou Municipal Science and Technology Bureau (201904010488). The sponsors also funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Conceptualization: Na Xu; Funding acquisition: Yan Wen; Xuan Zhou; Hong Qu; Zhenfang Liu and Na Xu; Investigation: Yan Wen; Yun Zeng; Lie Lin; Bihong Sun; Hongqian Zhu; Huiqing He; Xiaotao Wang; Waiyi Zou; Caifeng Zheng; Liling Zheng; Jinxiong Huang; Liping Pang; Jixian Huang; Yuming Zhang; Haiqing Lin; Zelin Liu; Wanshou Zhu; Qiang Wang; Xuan Zhou; Zhenfang Liu and Xin Du; Project administration: Na Xu; Resources: Yan Wen; Yun Zeng; Lie Lin; Bihong Sun; Hongqian Zhu; Huiqing He; Xiaotao Wang; Waiyi Zou; Caifeng Zheng; Liling Zheng; Jinxiong Huang; Liping Pang; Jixian Huang; Yuming Zhang; Haiqing Lin; Zelin Liu; Wanshou Zhu; Qiang Wang; Xuan Zhou; Xiaoli Liu; Zhenfang Liu and Xin Du; Supervision: Xiaoli Liu and Na Xu; Methodology: Na Xu and Hong Chen; Visualization: Hong Chen; Writing—original draft: Hong Chen; Writing—review & editing: Hong Qu and Na Xu. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Hong Chen, Yan Wen, Yun Zeng, Lie Lin, Bihong Sun, Hongqian Zhu, Huiqing He, Xiaotao Wang, Waiyi Zou, Caifeng Zheng, Liling Zheng, Jinxiong Huang, Liping Pang, Jixian Huang, Yuming Zhang, Haiqing Lin, Zelin Liu, Wanshou Zhu, Qiang Wang, Xuan Zhou, Xiaoli Liu, Hong Qu, Zhenfang Liu, Xin Du and Na Xu have nothing to disclose.
Ethical Approval
The study was reviewed by the Medical Ethics Committee of Southern Medical University Nanfang Hospital, who confirmed that the research qualified as exempt from medical ethical review under Article 1, “Conducting research using legally obtained public data or data generated through observation without interfering with public behavior,” as outlined in the exemption from medical ethics review instructions. Therefore, the application for exemption from medical ethics review for this study was approved. All enrolled patients provided written informed consent before beginning the survey, and the trial was conducted in line with the principles of the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (NCT05092048).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, H., Wen, Y., Zeng, Y. et al. Patient Versus Physician Perspective in the Management of Chronic Myeloid Leukemia During Treatment with Tyrosine Kinase Inhibitors. Oncol Ther 12, 131–145 (2024). https://doi.org/10.1007/s40487-023-00255-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40487-023-00255-2